ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Darshan N1, Sneha B Shetty2 *, Nayanatara AK2, Sheila R Pai2,Balachandra.A.Shetty1 Department of Medicine, A.J. Institute of Medical Sciences, Mangalore, Karnataka, India1 Departments of Physiology, Center for Basic Sciences, Kasturba Medical College (Manipal University) Bejai,Mangalore-575004, Karnataka, India2 |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The present study was aimed to evaluate the dispersion of QT interval, which is an index of inhomogeneity of ventricular repolarization and heart rate, and a measure of cardiac autonomic modulation in patients with clinical hypothyroidism .A total of 60 comprising of 30 healthy subjects who underwent TSH estimation for any other reasons and found to be normal were taken as control group and 30 patients who are diagnosed with clinical hypothyroidism, were selected from outpatient clinic of Department of General Medicine were taken as cases.. The ECG was recorded with BPL Cardiart 6108 T ECG Machine (BPL Manufacturing Company, BPL limited, India) and heart rate and QT dispersion was calculated. The mean QT intervals in all the leads of ECG differed significantly (p<0.05) between hypothyroid cases and normal control cases except in lead I . Mean QTc from the cases differed significantly (p<0.5) from the control group . Both QTmin and QTmax of cases and controls showed statistical significant difference (p=0.22 and P=0.014 respectively). Hypothyroid patients showed a highly significant increase(p<0.0001) in mean QTdis in respect to control group. In conclusion, the present study confirms the role of thyroid hormones on the cardiovascular functionality, especially on the ventricular repolarization and autonomic modulation of cardiac activity.
Keywords |
| QT dispersion ,Heart rate ,QT interval ,Hypothyroidism |
INTRODUCTION |
| Thyroid diseases are one of the commonly seen endocrine abnormalities, affecting about 10-15% of the adult female population [1]. It has long been recognized that hypothyroidism may cause cardiac pathologies, such as impaired cardiac contractility, decreased cardiac output, increased systemic vascular resistance, and cardiac electrical abnormalities [2,3]. Cardiac manifestations in hypothyroidism include reduction in cardiac output, stroke volume, heart rate, blood pressure and pulse pressure. Pericardial effusion is present in about 33% patients which later progresses to tamponade. Myocardial failure is uncommon.. But angina is not seen in these patients, because of low metabolic demands caused by their conditions [3]. There are also significant changes in modifiable atherosclerotic risk factors including hypercholesterolemia, diastolic hypertension, carotid intimal media thickness, and endothelial derived relaxation factor (nitric oxide), which accompany overt hypothyroidism [4]. Electrocardiographic changes such as sinus bradycardia, prolonged QT interval, prolonged AV conduction time, low voltage, and varying degrees of heart block are commonly recognized in hypothyroid patients [5].This study is taken up to assess the cardiac changes in terms of ventricular repolarization changes in fresh cases of hypothyroidism. T3 exerts its cellular actions through binding to thyroid hormone nuclear receptors. These receptor proteins mediate the induction of transcription by binding to thyroid hormone response elements in the promoter regions of positively regulated genes [6,7]. While bound to T3, Thyroid receptors induce transcription, and in the absence of T3 they repress transcription [8] . Negatively regulated cardiac genes such as β-myosin heavy chain and phospholamban are induced in the absence of T3 and repressed in the presence of T3 [9,10] . Thyroid hormone also has extranuclear nongenomic effects on the cardiac myocyte and on the systemic vasculature. These effects of T3 can occur rapidly and do not involve thyroid hormone response elements-mediated transcriptional events [11-17] The QTdispersion provides a potentially simple, inexpensive, noninvasive method of measuring underlying dispersion recovery of ventricular excitability [18]. Previous studies have shown that QT interval, QTc interval, QT dispersion, and the QTc dispersion significantly variations in patients with hypothyroidism [19-22]. The present study will help treating clinicians to have better understanding of cardiac functional variability under hypothyroid conditions. There is growing concern that, restoration of the heart to euthyroid state might adversely affect underlying ischemic heart disease. This study will address the QT interval variations in hypothyroid state and will give better picture of the functionality of the myocytes. This study by establishing the usual QT interval in hypothyroidism will aid the treating clinician in considering causes for long QT interval at times of ventricular arrhythmias. The aim of the study is to evaluate the dispersion of QT interval, i.e., an index of inhomogeneity of ventricular repolarization and heart rate, i.e., a measure of cardiac autonomic modulation in patients with clinical hypothyroidism, who are not having any complications nor have been hospitalized for any thyroid related or unrelated causes |
II. EXPERIMENTAL |
| A..Materials and Methods: |
| About 30 patients who are diagnosed with clinical hypothyroidism, as judged by elevated serum thyroid-stimulating hormone (TSH) levels (normal range 0.35–4.9 μIU/ml) were selected from our institution, outpatient clinic of Department of General Medicine. Patients who were found to be free from cardiovascular disease or any other major medical disorders, after assessing their medical history, physical examination, blood chemistry, hematology, and urine analysis were be considered for the study. |
| ïÃâ÷ Inclusion criteria:Body mass index lower than 30 kg/m2, diastolic arterial blood pressure lower than 90 mmHg, and systolic arterial blood pressure lower than 140 mmHg. Before inclusion in the protocol, a blood sample for the determination of TSH was obtained at 8’O clock, after an overnight fast. |
| ïÃâ÷ Exclusion criteria: Subjects were excluded if the physical examination revealed any abnormalities, or in presence of smoking habits, diabetes mellitus, or if they had received any drug treatment within the previous 3 months.Additionally, equal number of sex- and age-matched healthy subjects who underwent TSH estimation for any other reasons and found to be normal were selected from the medical record section of our institution and this group will be considered as control group. The study protocol was approved by the institutional ethics committee and informed consent form was taken from the patients who participate in the study. |
| B. Experimental Protocol |
| ïÃâ÷ ECG recording |
| ECGs with a duration of 10s was recorded with BPL Cardiart 6108 T ECG Machine (BPL Manufacturing Company, BPL limited, India), using the same system at 25mm/s paper speed and standardized at 0.1 V/mm. |
| ïÃâ÷ Measurement of Heart rate |
| Heart rate was calculated by ‘Box counting method’ in rhythm strip of lead II, where the small boxes (n) which measures 1mm is counted in the R-R interval and is measured manually with the formula: 1500/n. Its variability with respect to QT interval is analysed [5]. |
| ïÃâ÷ Measurement of QT interval and QT dispersion |
| QT intervals will be measured manually in all the 12 leads in blinded fashion from the onset of the QRS complex to the end of the T wave. |
 |
| When U waves are present, the QT interval will be measured to the nadir of the trough between the T and U waves. If the end of the T wave could not be identified, the lead will not included. Three consecutive QT intervals will be measured and averaged for each lead. A minimum of nine leads in which the QT interval could be measured will be required for QT dispersion to be determined. QT dispersion, defined as the difference between the longest and shortest QT intervals. With use of Bazett's formula, QT dispersion will be corrected (QTc) for heart rate.All ECGs will be analyzed twice by two observers, because of the known difficulties concerning definition of the end of the T wave. To minimize these confounding factors, two independent observers who were unaware of the clinical details will be asked to perform all measurements, and intra- and interobserver variability will be taken into consideration. |
III. STATISTICAL ANALYSIS |
| All data will be expressed as Mean ± SD(Standard Deviation). and compared using student t-test. Confidence intervals at the 95% level will be calculated for QT indices. Differences are considered significant when P<0.05. The Student's ttest will be used to compare the differences between groups and before and after treatment. |
IV. RESULTS |
| The mean age of the control and cases were 41.73 and 41.83 respectively and there is no statistical difference between the groups with respect to age (p=0.9). Mean TSH value of the hypothyroid group in the study was 22.75 ± 14.69 μIU/ml. Mean TSH value of the cases group was 1.60 ± 1.16 μIU/ml. Mean heart rate of the hypothyroid patients was 66 and that of normal patients selected under the study was 80 and the difference is statistically significant (p<0.005). The values are tabulated in table 1 |
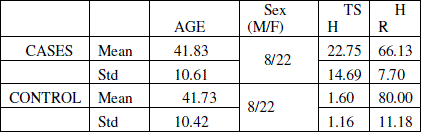 |
| Table 1: Tabulation of mean age, TSH and Hear rate (HR). TSH units μIU/ml |
| The readings from the control and cases ECG are tabulated in table 2 |
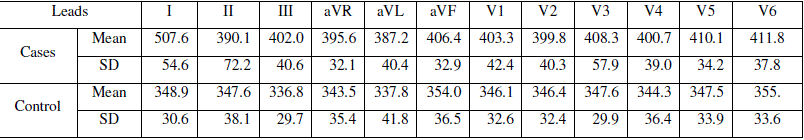 |
| Table 2: Tabulation of measurements from ECG leads, in milliseconds |
| The mean QT intervals in all the leads of ECG differ significantly between (p<0.05) hypothyroid cases and normal control cases except in lead I (p=0.12). Mean QTc from the cases (408±24) differed significantly (p<0.5) from the control group (394±27). Both QTmin and QTmax of cases and controls showed statistical significant difference (p=0.22 and P=0.014 respectively). Hypothyroid patients showed very significant increase in mean QTdis in respect to control group. Values are tabulated in table 3. |
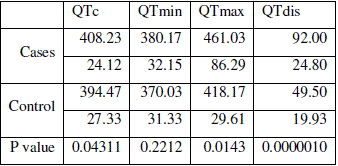 |
| Table 3: Tabulation of QT intervals and significance of difference |
V.DISCUSSION |
| The results of the study showed that there is reduction in heart rate and increase in QT intervals in the hypothyroid patients. This confirms that hypothyroidism is a state of decreased cardiovascular sympathetic activity. Our findings are consistent with prior studies [23-24] who have shown that there will be decreased adrenergic sensitivity in patients with hypothyroidism. Manhem P and et al have shown that during hypothyroidism venous and arterial noradrenaline were significantly higher as compared to euthyroidism [24], which is in correlation with our study stating prolonged QT intervals. Conflicting results about HRV have been reported by Polikar et al, Inuki et al [25-26, 16] . Cacciatori et al. showed an increased sympathetic influence in hypothyroidism [23], while Xing et al. showed a higher level of vagal tone. These conflicting results may be partially explained by the different selection of patients (number, age, gender, type, severity, and duration of hypothyroidism) in the various studies. However, the diversity of techniques used to monitor the sympatho-vagal imbalance may also be responsible for the contradictory results obtained. As shown by many studies in the literature [27, 28], a significant association is present between QTc dispersion and HRV. So, the contemporary evaluation of both these parameters should strengthen the significance of the results, and could lead to a firmer conclusion. The cardiac effects of hypothyroidism depend on the severity and duration of the disease. The occurrence of malignant arrhythmias is higher in long standing and severe hypothyroidism and in myxedema coma (where the mortality is more than 50% and often due to cardiac arrest). The evaluation of markers of arrhythmic risk, such as QTc dispersion (that can be easily monitored), will be helpful in evaluating the cardiac risk in these patients. The correlation of serum TSH with QT dispersion confirms that the severity of hypothyroidism plays an important role in these factors for arrhythmic risk. The increase in QT dispersion is closely associated with repetitive and life-threatening ventricular arrhythmias and has been shown to be an independent risk factor for sudden death [29,30]. However, the factors which may act on QT dispersion such as age, gender, myocardial ischemia, cardiac failure, diabetes, hypertension and electrolyte imbalance and some drugs, and the circadian pattern of QT dispersion, make its clinical use difficult [31]. Knowledge of QT dispersion is therefore clinically important. Hypothyroidism can affect cardiac structure [32,33]. The structural effects manifests clinically in hypothyroidism as an increase in myocardial echoreflectivity [34], and may be an explanation for the observed electrocardiographic abnormalities of hypothyroidism. |
VI. CONCLUSION |
| There is clear decrease in the heart rate in hypothyroid parients. QT dispersion interval is significantly prolonged in hypothyroid patients. This study has given a better understanding of thyroid hormones on the cardiovascular functionality, especially on the ventricular repolarization and autonomic modulation of cardiac activity. Thus study will be helpful for clinicians to correlate the thyroxin effects with the ventricular functionality. |
References |
|