e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
Gehan F Balata1,2*, Naser A ElSawy3,4, Mohamad AS Abourehab1,5, Nedaa Ali Karami6, Abdualrhmain Bahowirth6, Walaa Al Nemari6, Mashael Al Daajani6 and Ferdous Mohammed Turkistani6
1Department of Pharmaceutics, Faculty of Pharmacy, Umm Al-Qura University, Makkah, Saudi Arabia
2Department of Pharmaceutics, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt
3Department of Anatomy and Embryology Faculty of Medicine, Zagazig University, Egypt
4Department of Laboratory Medicine, Faculty of Applied Medical Sciences, Umm Al-Qura University, Saudi Arabia
5Department of Pharmaceutics, Faculty of Pharmacy, El-Minia University, El-Minia, Egypt
6Faculty of Pharmacy, Umm Al-Qura University, Makkah, Saudi Arabia
Received Date: 16/01/2017; Accepted Date: 30/01/2017; Published Date: 05/02/2017
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
Objective: The prevalence of liver diseases in Saudi Arabia is relatively high, and the mortality rates are significant. So, the aim of the study was to modify the physicochemical and hence biological characteristics of trans-resveratrol, a hepatoprotective agent, through the formation of ternary β-cyclodextrin/soybean lecithin complexes by solvent evaporation and freeze drying techniques using a 23-full factorial design methodology.
Methods: Solubility of resveratrol in presence of (0 M to 0.02 M) β-cyclodextrin with and without 0.5% lecithin was studied. The prepared complexes were evaluated by dissolution study, scanning electron microscopy, differential scanning calorimetry and x-ray powder diffractometry. The effect of β-cyclodextrin and soybean lecithin concentration and preparation method, independent variables, on the amount of resveratrol released after 10 min (Q10) and dissolution efficiency after 60 min (DE60), dependent variables, were studied using contour plot and interaction surface plot. Finally, the therapeutic effectiveness of the optimized resveratrol complex (F8) compared to a commercial silymarin product (Legalon)© was investigated biochemically and histopathologically in rats after carbon tetrachloride (CCl4)-induced hepatotoxicity.
Results: The results revealed that the addition of lecithin increased the complexation efficiency and the stability constant by about 3 folds. Physicochemical characterization using scanning electron microscopy, differential scanning calorimetry and x-ray powder diffractometry revealed resveratrol complexation within β-cyclodextrin cavity with reduction in drug crystallinity. The contour and interaction charts indicated that the method of complex preparation was the most important factor affecting dissolution performance of resveratrol from its complexes followed by lecithin concentration and then β-cyclodextrin concentration. The optimized resveratrol complex (resveratrol: β-cyclodextrin: lecithin, 1:2.5:0.5 w/w) had a pronounced therapeutic effect against hepatic damage when compared with the commercial silymarin product (Legalon)©.
Conclusion: Ternary resveratrol complex with cyclodextrin and lecithin may be a good alternative medicine for treatment of liver damage.
Resveratrol, β-Cyclodextrin, Lecithin, Ternary complex, Carbon tetrachloride, Induced hepatotoxicity
The use of naturally occurring phytochemicals, in the amelioration of illness has recently gained considerable popularity [1,2]. Natural products have wide applications among Saudi population as they believe that natural products have numerous health benefits with no harmful side-effects. Liver diseases are prevalent in Saudi Arabia, and the mortality rates are significant. Al- Zahim et al. conducted a study among Saudi liver disease patients about the use of natural products to treat their liver disease and they found that 76.6% of the participants were satisfied with using natural products to help control their disease [3]. Grape is the most-widely cultivated fruit in the world. Grape skin contains polyphenol (resveratrol) which has high antioxidant activity. Recently, extracts containing resveratrol have been already used for treatment of many diseases which include heart disease, hypercholesterolemia and inflammation. It could limit the spread of cancer cells and it is effective as a hepatoprotective agent [4]. However, resveratrol has low bioavailabilty in humans due to extensive metabolism by the intestinal epithelium, low solubility, as well as photosensitivity during formulation development [5,6]. Many approaches have been developed to increase resveratrol bioavailabilty, including micronization, solubility improvement by complexation with hydroxypropyl-β-cyclodextrin and loading resveratrol onto lipid-core nanocapsules [7-9].
Inclusion complexation with cyclodextrins is used to modify the physicochemical properties of drugs. Previous literature reported the improved solubility and hence bioavailability of many poorly water soluble drugs by β-cyclodextrin complexation [10,11]. The accommodation of drugs (guest molecules) into cyclodextrin cavity (host molecules) depends on the physicochemical properties of both guest and host molecules. One of the disadvantages of inclusion complexation is high volume of the dosage form depending on the amount of cyclodextrin required. One approach to use smaller amounts of cyclodextrin is to increase the solubilisation efficiency and consequently the complexation effectiveness by adding small amounts of water-soluble polymers to the system [12,13]. Previous studies stated that the addition of small amounts of water soluble polymers such as polyvinyl pyrrolidone, hydroxyl propyl methyl cellulose and tween 80 during the formulation of drug - cyclodextrin complexes resulted in increasing the drug bioavailability while reducing the amount of cyclodextrin by up to 80% [14]. So, the aim of this study was to modify the physicochemical and hence biological characteristics of resveratrol through the formation of ternary resveratrol-β-CD complexes with soybean lecithin.
Materials
Trans-resveratrol was purchased from Xian Lukee Bio-Tech Co., Ltd., (Xi’an, Shaanxi Sheng, People’s Republic of China). Beta cyclodextrin (β-CD), soybean lecithin, and olive oil were purchased from Yarrow Chem Pvt. Ltd, Mumbai. The commercially available silymarin product (Legalon capsules) ©, each capsule contains dry extract of milk thistle fruits 86.5 mg to 93.35 mg equivalent to silymarin 70 mg. Ethyl alcohol and carbon tetrachloride (CCl4) were purchased from BDH Chemicals, UK.
Factorial Design
Eight batches (F1-F8) of resveratrol inclusion complexes were prepared based on the 23-factorial design in which three independent factors i.e. concentration of β-CD (A) and concentration of soybean (B) at two levels, i.e. high (+) and low (-) and preparation method. The concentration of β-CD was 2.5% (-) and 5% (+) whereas the concentration of soybean lecithin was 0.5% (-) and 1% (+) and two preparation methods were used: solvent evaporation (-) and freeze drying (+). In addition, the amount of drug released after 10 min (Q10) and dissolution efficiency after 60 min (DE60) were taken as response parameters as the dependent variables. The investigated formulation variables are represented in Figure 1.
Preparation of resveratrol inclusion complexes by solvent evaporation
Batches 1-4 (Table 1) were prepared by the solvent evaporation method as follows. Specified amounts of resveratrol together with β-CD and soyabean lecithin were stirred with 20 mL ethanol at 800 rpm (Magnetic Stirrer WU-04807-30, Cole- Parmer International, 625 East Bunker Court Vernon Hills, USA) for 1 h. Then the beaker was placed in a fume hood at room temperature (25°C ± 1°C) for 24 h to allow the solvent to gently evaporate. The dried mass was passed through a- 80 mesh sieve and the resulting inclusion complex powder was kept in a desiccator at 25°C ± 1°C and protected from light till use [15].
| Batch | Ratio | Resveratrol | β-CD concentration | soybean lecithin concentration | Preparation method |
|---|---|---|---|---|---|
| F1 | 1:2.5:1 | 1.11 | 2.78 | 1.11 | Solvent evaporation |
| F2 | 1:5:0.5 | 0.77 | 3.85 | 0.385 | Solvent evaporation |
| F3 | 1:5:1 | 0.7 | 3.6 | 0.7 | Solvent evaporation |
| F4 | 1:2.5:0.5 | 1.25 | 3.125 | 0.625 | Solvent evaporation |
| F5 | 1:2.5:1 | 1.11 | 2.78 | 1.11 | Freeze drying |
| F6 | 1:5:0.5 | 0.77 | 3.85 | 0.385 | Freeze drying |
| F7 | 1:5:1 | 0.7 | 3.6 | 0.7 | Freeze drying |
| F8 | 1:2.5:0.5 | 1.25 | 3.125 | 0.625 | Freeze drying |
Table 1: Composition of the different resveratrol ternary inclusion complexes.
Preparation of resveratrol inclusion complexes by freeze drying
Batches 5-8 (Table 1) were prepared by freeze drying as follows. β-CD and soyabean lecithin were dissolved in 50 mL of 50% v/v ethanol. Resveratrol was added and stirred until a clear solution was obtained. The solution was frozen at -40°C for 24 h and lyophilized in a freeze dryer (DFU-1200, Tokyo Rikakikai Co., Ltd., Japan) at -40°C for 48 h. The dried mass was passed through a-80 mesh sieve and the resulting inclusion complex powder was kept in a desiccator at 25°C ± 1°C and protected from light till use [15].
Phase solubility study
Phase solubility was studied according to the method described by Higuchi and Connors [16]. Excess amount of resveratrol 20 mg was added to 5 mL dist. water containing different molar concentrations (0 M to 0.02 M) of β-CD with and without 0.5% lecithin. Then the dispersions were transferred to a thermostatic shaker water bath (LSB-030S, Daihan Labtech Co. Ltd., New Delhi, India) maintained at 25°C for 72 hours. The solubility of reseveratrol alone in dist. water was determined as well. After equilibration, the samples were filtered through a 0.45 μm filter (Millipore, MA, USA) and analyzed spectrophotometrically at 306 nm [17].
Phase solubility diagram was constructed by plotting the molar concentration of resveratrol versus the molar concentration of β-CD in absence and presence of 0.5% lecithin. For the 1:1 drug/β-CD complex, the complexation efficiency and the apparent stability constant could be estimated from the slope of the phase solubility diagram as follows: [18].
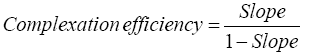
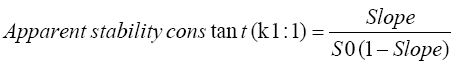
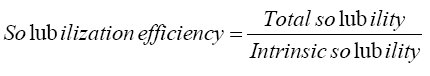
Where, slope was the slope of the phase solubility diagram and S0 was the equilibrium solubility of resveratrol in dist. Water without any additives.
Determination of resveratrol content
Specified amount of each inclusion complex containing equivalent to 25 mg of resveratrol was taken and placed in a 5 mL volumetric flask with 50% v/v ethanol. The samples were sonicated using an ultrasonic sonicator for 30 min. The solution was filtered through a 45 μm filter (Millipore, MA, USA), diluted with 50% v/v ethanol and analyzed spectrophotometrically at 306 nm. Each sample was carried out in triplicate [17].
The percent drug content was calculated as follows:
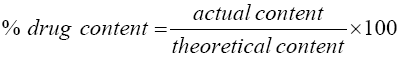
Characterization of resveratrol inclusion complexes Scanning electron microscopy (SEM)
The surface morphology of two selected resveratrol inclusion complexes compared with pure resveratrol and β-CD was studied by SEM (JSM-6360A, Jeol, Tokyo, Japan). Samples were fixed onto an aluminum stub using double sided adhesive tape and coated with gold-palladium in vacuum in order to make them electrically conductive. The micrographs at different magnifications were recorded by working at an excitation voltage of 15 kV [19].
Differential scanning calorimetery (DSC)
DSC analysis of the selected resveratrol inclusion complexes compared with the individual ingredients was performed using differential scanning calorimeter (Model DT-60, Shimadzu, Japan) to determine the physical state of the drug in the prepared complexes. Under nitrogen atmosphere of flow rate 80 mL/min, 5 mg of the samples were sealed in a flat-bottomed aluminum pan, heated in a temperature range of 30°C to 400°C and a constant rate of 10°C min-1. The empty aluminum pan was used as a reference [19].
X-ray powder diffractometery (XRPD)
XRPD study was performed for the selected resveratrol inclusion complexes compared with the individual ingredients using an X’Pert PRO diffractometer (PAnalytical, USA) to detect the physical form of resveratrol in the prepared complex. The diffraction pattern was measured at a 2-theta angle range of 3°to 60° [19].
in vitro dissolution studies
The in vitro dissolution studies were carried using USP Dissolution Tester apparatus II, paddle type, (SP6-400, G.B. CALEVA Ltd., Dorset, England) at paddle rotation speed of 100 rpm in 900 mL dist. water at 37°C. A sample of 25 mg resveratrol or its equivalent of the prepared complexes were filled into empty hard gelatin capsules (size 0) and placed into the dissolution medium. An aliquot of 5 ml was collected at 5, 10, 15, 20, 30, 40, 50 and 60 min and replaced with fresh dissolution medium. The samples were filtered through filtered through a 0.45 μm filter (Millipore, MA, USA) and analyzed spectrophotometrically at 306 nm. All experiments were carried out in triplicate [20].
In order to compare the dissolution properties of different resveratrol formulations, percentage of resveratrol released after 10 min (Q10), dissolution efficiency after 60 min (DE60) and dissolution enhancement ratio were calculated as follows:
Dissolution efficiency (DE) can be calculated from the area under the dissolution curve between time points t1 and t2 and expressed as percentage of the area of the rectangle described by 100% dissolution, y100, over the same time period [21].
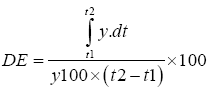
Where, y is the percentage of dissolved drug.
The dissolution enhancement ratio (DER) can be obtained by dividing the dissolution efficiency after 60 min for resveratrol complex by the dissolution efficiency for pure resveratrol.
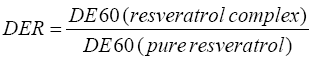
Pharmacological Study
Resveratrol formulation with optimal dissolution parameters was selected for in vivo study and compared with commercially available silymarin product (Legalon)©.
Animals
All animal care and procedures were conducted according to the Guidelines for Animal Care and Treatment of the European Community and was approved by the Institutional Animal Ethical Committee, Faculty of Pharmacy, Umm Al Qura University. Fifty male albino rats weighing 180 g to 200 g was housed at 25°C to 30°C with free access to food and water and were allowed to acclimatize for 1 week before starting the experiment.
Basal diet
The basal diet was prepared and consisted of 20% protein(casein), 10% sucrose, 4.7% corn oil, 2% choline chloride, 1% vitamin mixture, 3.5% salt mixture and 5% fiber (cellulose). The remainder was corn starch.
Experimental design
Fifty rats were divided into five groups of ten animals each (n=10). The study was based on the use of carbon tetrachloride (CCl4)-induced hepatotoxicity model as it is one of the most frequently used models to study the acute liver injury [22].
Group-I (normal control): it was used as normal control rats and they were given 1 mL saline orally daily for 14 days.
Group-II (positive control): each rat received 2.5 mL/kg body weight of CCl4 solution in olive oil (1:1) v/v ratio IP once [20].
Group III: each rat received 2.5 mL/kg body weight of CCl4 solution IP once. 72 h later, 75 mg/kg. [23] Pure resveratrol (3 ml suspension in CMC) was given orally once a day for 11 successive days.
Group IV: each rat received 2.5 mL/kg body weight of CCl4 solution IP once. 72 h later, equivalent to 75 mg/kg resveratrol (F8) (3 ml suspension in CMC) was given orally once a day for 11 successive days.
Group V: each rat received 2.5 mL/kg body weight of CCl4 solution IP once. 72 h later, equivalent to 75 mg/kg silymarin (Legalon)© (3 ml suspension in CMC) was given orally once a day for 11 successive days.
On the 14th day, two hours after treatments, blood samples were obtained via retroorbital sinus plexus and then rats were sacrificed. Blood was left to clot at room temperature and the serum was obtained by centrifugation at 4000 rpm for 15 min and kept at -20°C for further biochemical analysis. Portions of liver tissues were obtained and immediately embedded in 10% formalin and processed for histopathological assay. Tissue sections of liver were stained with hematoxylin and eosin (H&E). Biochemical analysis includes liver function test (measured as AST, ALT, ALP, BILT, TP and ALB) and kidney function test (measured as serum urea and creatinine). Biochemical assays were carried out using commercially available kits
Histopathological analysis of liver
For Histopathological studies, the liver tissues were obtained from rats of different groups, washed with ice-cold normal saline, and immediately fixed in phosphate buffered neutral 10% formalin. After proper fixation, the specimens were dehydrated in graded ethyl alcohol (50% to 100%), cleared in xylene, embedded and casted in paraffin. Thin tissue paraffin sections (5 μm thickness) were prepared and stained with routine hematoxylin and eosin (H&E) in addition to staining with toluidine blue stain. Specimens were examined under microscope for histopathological changes in the liver tissues, and photographed at a different magnification power. The histological examination was done in Histology lab in anatomy department, faculty of medicine Umm Al-Qura University, Saudi Arabia.
Phase Solubility Study
Resveratrol was slightly soluble in water (8.24 × 10-5 M). The solubility of resveratrol was significantly increased to 0.00094 M in presence of β-CD with a solubilization efficiency of 11.4. Addition of 0.5% lecithin w/v as a third auxiliary agent further increased resveratrol solubility to 0.002 M with a solubilization efficiency of 24.3, representing 2.1 folds’ increase (Table 2).
| Carrier | Complexation efficiency | Stability constant (K1:1) M-1 | Solubilization efficiency |
|---|---|---|---|
| β-CD | 0.05 | 611.54 | 11.4 |
| β-CD/0.5% lecithin | 0.153 | 1860 | 24.3 |
Table 2: Data of phase solubility study of resveratrol in different molar concentrations of β-CD with and without lecithin.
The phase solubility diagram of resveratrol in different molar concentrations of β-CD with and without 0.5% lecithin w/v is shown in Figure 2. The aqueous solubility of resveratrol was increased linearly with the increase of β-CD concentration depicting an AL type phase solubility diagram according to Higuchi and Connors. The observed increase in solubility was attributed to the formation of a 1:1 M inclusion complex [24]. The extent of complexation in aqueous media is characterized by the apparent stability constant K1:1. The calculated value for resveratrol-β-CD stability constant (K1:1) was 611.54 M-1 which was in agreement with the previously reported range (50 M-1 to 2000 M-1) by Cannors [16]. The calculated complexation efficiency of resveratrol with β-CD was 0.05. However, the addition of small amount of lecithin to the β-CD system had further enhanced the complexation and solubilizing efficiency of β-CD. The stability constant K1:1 and complexation efficiency of the ternary complex were 1860 M-1 and 0.153, respectively, showing 3-folds increase in both values. This result confirmed our assumption that the addition of small amount of lecithin will decrease the required amount of drug complex during formulation. Loftsson 2005, reported that as the drug cyclodextrin complexation efficiency increased, the drug complex bulk dosage decreased [25].
The enhanced cyclodextrin complexation by the addition of small amount of lecithin was ascribed to the synergistic effect between these components. It was reported that phospholipids act as surface active agents that improves drug wettability with the advantage of being nontoxic and biocompatible [26]. Loftsson and coworkers explained the synergistic effect of water soluble polymers on cyclodextrin complexation by the ability of polymers to stabilize micelles and other types of aggregates, reduce cyclodextrin mobility and increase the solubility of complexes by changing the hydration properties of cyclodextrin molecules [27].
Determination of Resveratrol Content
The % drug content of all the prepared inclusion complexes was found to be between 98.6% and 99.60% indicating that the drug is uniformly dispersed within the prepared complexes.
Characterization of Resveratrol Inclusion Complexes
Scanning electron microscopy (SEM)
The surface morphology of the selected resveratrol complexes (F4 and F8) compared to pure resveratrol and β-CD is illustrated in Figure 3. Pure resveratrol exhibited a rod-shaped form with smooth surfaces (Figure 3A), while β-CD showed a cubic shape with smooth surfaces (Figure 3B). On the other hand, the formulations (F4 and F8) showed a different morphology than the individual ingredients and exhibited a change in the crystal structure of the drug (Figure 3C and 3D) which may be ascribed to the complexation process [28].
Differential scanning calorimetery (DSC)
DSC thermograms of the individual ingredients and some selected resveratrol complexes are shown in Figure 4. Resveratrol showed a sharp endothermic peak at 272.7°C (ΔH= -45.8 J/g) corresponding to its melting point [29]. β-CD had a broad endothermic peak at 114.65°C (ΔH= -85.12 J/g) corresponding to the evaporation of water included in the cyclodextrin cavity [29]. Lecithin exhibited one endothermic peak at 202.13°C (ΔH= -18.26 J/g). Different resveratrol complexes showed only one broad peak at 122-137°C. However, the endothermic peaks in case of cyclodextrin complexes prepared by freeze drying (F7 and F8) showed marked reduction in the heat of fusion when compared to those prepared by solvent evaporation (F3 and F4). These changes may be explained by complex formation and crystalline change of resveratrol into amorphous state which was more significant in resveratrol complexes prepared by freeze drying [24]. Gidwani and Vyas reported that the driving force for drug- cyclodextrin complexation involves the removal of water molecule from cyclodextrin hydrophobic cavity and formation of Vander Waal forces and hydrogen bond interactions [30].
X-ray powder diffractometery (XRPD)
XRPD of the individual ingredients and some selected resveratrol complexes are shown in Figure 5. Both pure resveratrol and β-CD exhibited multiple diffraction peaks in the region 6.6° to 32° with high intensity (13.8% to 100%) indicating their crystalline nature, while lecithin showed amorphous nature. Different resveratrol complexes showed disappearance of resveratrol peaks with presence of some new peaks at different diffraction angles. However, resveratrol complexes prepared by freeze drying (F7 and F8) showed marked reduction in the intensity of diffraction peaks indicating more reduction in drug crystallinity compared to those prepared by solvent evaporation. The results of XRPD were parallel with that obtained by DSC results.
in vitro dissolution Studies
Dissolution profiles of different resveratrol complexes compared with pure resveratrol are demonstrated in Figure 6. The calculated dissolution parameters for different resveratrol complexes compared with its pure form are summarized in Table 3. Pure resveratrol exhibited slow dissolution rate (Q10=5.8% ± 1.2%) with small amount of drug released at the end of 60 min (DE60=10.6% ± 1.1%). This result was attributed to hydrophobic drug nature and hence poor wettability, as well as particle agglomeration during the dissolution process [17]. The dissolution profiles of different resveratrol complexes showed significant improvement in the rate and extent of drug dissolution which could be explained on the basis of complex formation and consequently increased drug wettability by the augmented effect of β-CD and lecithin. The synergistic surface active properties of both β-CD and lecithin can reduce the interfacial tension between resveratrol particles and the dissolution medium, leading to a greater rate of dissolution [31]. Another contributing factor to enhanced drug dissolution is reduction in drug crystallinity which was confirmed through the characterization studies [32].
| Batch | Q10 | DE60 | DER |
|---|---|---|---|
| Pure resveratrol | 5.8 ± 1.2 | 10.6 ± 1.1 | - |
| F1 | 17.2 ± 0.5 | 45.6 ± 0.7 | 4.3 |
| F2 | 28.5 ± 0.6 | 57.4 ± 1.00 | 5.4 |
| F3 | 36.7 ± 1.5 | 70.1 ± 1.8 | 6.6 |
| F4 | 54.5 ± 4.00 | 75.1 ± 2.1 | 7.1 |
| F5 | 30.6 ± 1,1 | 79.6 ± 1.5 | 7.5 |
| F6 | 50.2 ± 1.7 | 83.4 ± 4.1 | 7.9 |
| F7 | 80.6 ± 5.6 | 91 ± 7.5 | 8.6 |
| F8 | 100 ± 3.5 | 95.8 ± 5.1 | 9 |
Table 3: Dissolution parameters of different resveratrol complexes compared with pure resveratrol.
However, resveratrol complexes prepared by freeze drying showed higher dissolution properties than those prepared by solvent evaporation. The formulation F8 showed 100% dissolution within 10 min. Superiority of freeze drying technique in dissolution enhancement of resveratrol can be strongly supported by the characterization results. Badr-Eldin and coworkers explained the marked increase in resveratrol dissolution from complexes prepared by freeze drying on the basis of complete inclusion of the drug in the cyclodextrin cavity during freeze drying process resulted in the formation of a solid solution of the drug and hence its particle size is reduced to the molecular size leading to fast dissolution [33].
To statistically understand the effects of various formulation variables, β-cyclodextrin and soybean lecithin concentration (X1, X2) and preparation method (X3 for freeze drying, X4 for solvent evaporation), on percentage of resveratrol released after 10 min (Q10) and dissolution efficiency after 60 min (DE60), a regression analysis and a three-factored two-leveled factorial design of experiments were performed using the Minitab 17 software and the results are shown in Table 4 and Figures 7-9.
Figure 8: Contour plots showing the effect of β-CD and lecithin concentrations on the dependent factors; Percentage of resveratrol released after 10 min (Q10) and dissolution efficiency after 60 min (DE60); A and B: for complexes prepared by freeze drying (method 1); C and D: for complexes prepared by solvent evaporation (method 2).
| Multiple regression analysis | |||||
|---|---|---|---|---|---|
| Response | A | y1 | y2 | y3 | y4 |
| Q10 | 65.3 | -0.79 | -8.5 | 0 | -31.1 |
| P-value | 0.009 | 0.939 | 0.431 | - | 0.018 |
| DE60 | 87.45 | -0.73 | -3.18 | 0 | -25.4 |
| P-value | 0.000 | 0.845 | 0.505 | - | 0.043 |
| Analysis of variance | |||||
| Degree of freedom | Sum of squares | Mean square | P>F | R2 | |
| Q10 | 7 | 16724.3 | 2389.19 | 0.000 | 0.9999 |
| DE60 | 7 | 5835.21 | 833.602 | 0.000 | 0.9921 |
Table 4: Results of multiple regression analysis and analysis of variance for the effect of different formulation variables on the investigated responses.
The multiple regression analysis can be interpreted using the following equation:
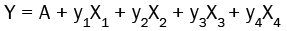
Where, Y is the response to be predicted. A is the intercept of regression equation. y1, y2, y3 and y4 are coefficients of changing the variables linearly. The regression models showed prediction efficiencies of 95.46% and 89.52% for the percentage of resveratrol released after 10 min (Q10) and dissolution efficiency after 60 min (DE60), respectively. The concentration of both β-cyclodextrin and soybean lecithin had a nonsignificant (P>0.05) negative effect on the percentage of resveratrol released after 10 min (Q10) and dissolution efficiency after 60 min (DE60). On the other hand, solvent evaporation method had a significant negative effect (P<0.05) on the dissolution performance of resveratrol from its complexes when compared with freeze drying technique.
The results of analysis of variance revealed that the eight resveratrol complexes exhibited strong significant differences among the values of the dependent variables by changing the levels of the investigated factors (Table 4). In order to verify the results of ANOVA analysis, residual plots were obtained. Since the residuals looked normally distributed and randomly patterned, the analysis was considered acceptable (Figure 7).
Contour plots
The two-dimensional contour diagrams were employed to understand the variation of the percentage of resveratrol released after 10 min (Q10) and dissolution efficiency after 60 min (DE60) at the highest and lowest values of the independent variables (Figure 8). At low value of β-CD, increasing lecithin concentration showed a negative influence on the rate of drug release (Q10) and extent of drug dissolution (DE60). The same results were obtained at high β-CD value. It is worthy to mention that lecithin concentration had stronger influence on the dissolution performance of resveratrol from its ternary complexes rather than β-CD concentration. This result could be ascribed to increase lecithin concentration decreased the affinity of resveratrol to β-CD cavity with subsequent decrease in the overall dissolution performance.
Interaction among variables
The relationship between different formulation variables, β-CD and lecithin concentrations and preparation method, were investigated (Figure 9). An interaction means the abolished effect of one variable at a certain value of the other variable. In these plots, degrees of interaction can be related to non-parallelism and the more non-parallel lines are the stronger the interaction [34].
The plots at the upper right shows the effect of β-CD concentration on the percentage of resveratrol released after 10 min (Q10) (A) and dissolution efficiency after 60 min (DE60) (B) when using two preparation methods. The effect of preparation method was more superior in enhancing dissolution properties (Q10, DE6) than the concentration of β-CD. The next plot showed strong interaction between β-CD concentration and lecithin concentration. At low β-CD concentration, increasing lecithin concentration negatively affected the dissolution properties of resveratrol from its complexes. On the other hand, at high β-CD concentration, increasing lecithin concentration resulted in some increase in resveratrol dissolution. The plot at the downright shows the effect of lecithin concentration on the percentage of resveratrol released after 10 min (Q10) (A) and dissolution efficiency after 60 min (DE60) (B) when using two preparation methods. There is no interaction between the two variables regarding their effect on the rate of drug release (Q10), however, there is some interaction between both variables when affecting the dissolution efficiency (DE60). Thus, the variables could be ranked as lecithin concentration > preparation method > β-CD concentration for their effect on the rate of drug release (Q10).
However, preparation method >lecithin concentration >β-CD concentration was their ranking for the effect on dissolution efficiency after 60 min (DE60).
Pharmacological Study
Biochemical analysis
The results of liver and kidney functions tests are summarized in Table 5. It is clear that administration of CCl4 resulted in a highly significant (P<0.001) elevation of hepatospecific serum markers ALT, AST, ALP and total bilirubin and reduction in the total protein in comparison with the normal control rats. In addition, there was a highly significant (P<0.001) elevation in renal serum markers creatinine and urea.
| Commercial silymarin | Cyclodextrin complex (F8) | Pure resveratrol | Positive control | Normal control | Parameter |
|---|---|---|---|---|---|
| 261.55±17.69bc | 238.9±18.52bc | 342.03±27.14ab | 446.04±28.89a | 224.39±18.16 | ALP (U/L) |
| 87.78±11.70abc | 73.34±9.15bc | 124.12±7.52ab | 162.62±13.48a | 63.22±10.66 | ALT (U/L) |
| 170.02±17.61bc | 161.48±16.28bc | 265.8±20.80ab | 315.42±21.02a | 152.22±14.87 | AST (U/L) |
| 0.382±0.03bc | 0.328±0.05bc | 1.516±0.17ab | 2.888±0.18a | 0.292±0.03 | Creatinine (mg/dL) |
| 52.52±2.48abc | 51.4±1.86bc | 66.38±3.72ab | 76.96±2.65a | 47.12±2.17 | Urea (mg/dL) |
| 0.65±0.07bc | 0.636±0.078bc | 1.23±0.13ab | 1.762±0.17a | 0.584±0.05 | BILT (mg/dL) |
| 7.04±1.11abc | 8.1±0.58bcd | 4.86±0.35a | 3.74±0.52a | 8.68±0.49 | TP (g/dL) |
| 3.764±0.30bc | 4.084±0.26bc | 2.452±0.31ab | 1.714±0.45a | 4.42±0.39 | ALB (g/dL) |
Values are mean ± SEM; n=10. a Significant difference vs normal control group; b Significant difference vs positive control group; c Significant difference vs resveratrol group
Table 5: Therapeutic effect of resveratrol ternary complex (F8) compared with pure resveratrol and commercial silymarin on serum hepatic and renal function tests in CCL4 treated male albino rats.
The hepatotoxic effect of CCl4 is ascribed to its metabolism by the microsomal cytochrome P450 (CYP) in mammalian liver into lethal reactive metabolites such as trichloromethyl (CCl3•) and peroxy trichloromethyl (CCl3OO•) radicals which result in peroxidation of a variety of biologically important cellular molecules, such as proteins, lipids, and nucleic acids. This causes impairments of liver function leading to hepatocellular damage [20].
On the other hand, treatment with either resveratrol formulations or commercial silymarin for successive 14 days highly significantly (P<0.001) alleviated these adverse reactions of CCl4. Comparing the therapeutic effectiveness of the three formulations (pure resveratrol, resveratrol complex (F8) and commercial silymarin), there was a highly significant difference (P<0.001) between pure resveratrol and both resveratrol complex (F8) and commercial silymarin. However, there was no significant difference (P>0.05) between resveratrol complex and commercial silymarin suggesting that both formulations have the same alleviating effect. The beneficial effect of resveratrol in treating hepatic damage is ascribed to its polyphenolic content which has potent anti-oxidant and anti-inflammatory properties, mediates induction of anti-oxidant enzymes, and decreases hepatic lipid peroxidation [35]. It was reported that resveratrol increases hepatic glutathione content, scavenges free radicals, and induces enzymes of phase II hepatic metabolism. Additionally, it inhibits the transcription factor nuclear factor-kappa B (NF kB), which induce inflammatory reactions as well as it reduces the expression of several pro-inflammatory cytokines [36,37]. The mechanism of therapeutic effect offered by silymarin against hepatic damage is also due to its high phenolic content which has strong antioxidant activity [38]. It prevents the penetration of the liver toxin into the hepatocytes by altering the outer structure of the cell membrane [39] increases the ribosomal protein synthesis, thereby stimulating the formation of new hepatocytes [40] and inhibits the synthesis of the chemical inflammatory mediators [41].
The study revealed that commercial silymarin had a more effective role than pure resveratrol in treating liver damage which was in agreement with previously reported findings by Kumar and coworkers [42]. However, the complexed resveratrol (F8) showed comparable hepatotherapeutic activity with the commercial sylimarin product. This observed enhancement in the hepato-therapeutic effect of resveratrol complex (F8) when compared with pure resveratrol was probably due to enhancement in drug dissolution by the synergistic effect of β-CD and lecithin which promotes faster release of the drug in body fluids and hence a rapid partitioning into the intestinal membrane and simultaneous absorption [20]. Similar results were reported by Kumari and coworkers [43].
Histopathological analysis
The biochemical observations were paralleled by histopathological findings in rat liver both in the case of CCl4 and treatment groups. Microscopic examinations for the liver tissues of rats from different groups using different stains are shown in Figures 10 to 12.
Figure 10 shows the histological examination upon using H&E stain, where, liver sections of the normal control group rats (10-I) showed the normal histological structure of hepatic lobule; normal lobular architecture with a central vein and radiating hepatic cords. It reveals normal hepatic cells with prominent nucleus and nucleolus.
While liver sections from rats treated with CCl4 (10-II) shows complete loss of hepatic architecture, as it revealed steatosis, dilated portal spaces, focal hepatic necrosis associated with degenerative changes, marked vacuolization, hyper-chromatosis and necrosis of the hepatocytes; centrilobular necrosis. Ballooning of the hepatocytes could be easily detected, in addition to severe inflammation in the portal zone accompanied by adjacent lobular spell over, narrowing of hepatic sinusoids and cellular infiltrations and fibrosed blood vessel. The liver damage effects induced by CCl4 could be mainly attributed to its oxidative stress mechanism, as the metabolites of CCl4 can initiate direct oxidative damage to lipids, proteins, and DNA [44].
Liver specimens from rats treated with pure resveratrol (10-III) shows moderate portal tract inflammation, moderate degenerative changes with infiltration of portal tracts with mono-nuclear cells. Necrotic hepatocytes focal areas were also detected. On the other hand, liver sections from rats treated with the optimized resveratrol formula, F8, (10-IV) shows minimal degenerative changes, minimal infiltration of portal tracts with mono-nuclear cells. No cell necrosis could be observed. It shows more or less normal lobular structure comparable to the normal control group. The hepatic cells may be restored to normal state.
Liver section in reference treated group (10-V) shows mild degenerative changes with infiltration of portal tracts with mononuclear cells. Mild inflammation and congestion of the central vein could be observed, which are somewhat higher than that could be observed in rats treated with the optimized formula. At high magnification, the hepatocytes showed swelling and foamy appearance besides scattered fibrotic tissues which could be seen.
Figure 11 represents the microscopic examination using Toluidine Blue (TB) stain. It could be noticed that, liver sections from normal control rats (11-I) showed the normal hepatocytes, central vein, and portal tracts. The micrograph for liver sections from rats treated with CCl4 only-diseased group- shows stasis, degenerative changes, and mast cell infiltration (11-II).
While, liver sections of rat treated with pure resveratrol (11-III) revealed moderate degenerative changes with infiltration of portal tracts with mono-nuclear cells. Apparent normal hepatic lobule was noticed in liver from rats treated with the optimized resveratrol formula, F8, revealed minimal histopathological changes (11-IV). While the micrograph of the liver sections of rats treated with reference showed mild degenerative changes with infiltration of portal tracts with mono-nuclear cells (11-V). The obtained results using TB stain augmented the results obtained using H&E stain. These findings were in agreement with Girish et al. who supported the use of active phytochemicals of silymarin against toxic liver injury, which may act by preventing the lipid peroxidation and augmenting the antioxidant defense system or regeneration of hepatocytes [45].
The histopathological finding by periodic acid chief (PAC) staining showed that no histochemical reaction for glycogen accumulations in hepatocytes of normal control group with normal hepatocytes central vein, portal tracts, and no histochemical reaction for glycogen accumulations (12-I), however the hepatocytes in second group (diseased group), associated with defective metabolism of glycogen. Glycogen accumulates in the cytoplasm of hepatocytes causes an ‘osmotic load’ and increase water content and grossly, the liver is pale but certainly not to the extent as a severe fatty liver. Microscopically, affected hepatocytes are swollen, pale and vacuolated, cytoplasm is sometimes referred to as ‘ground glass’ (cloudy swelling) (12-II). Meanwhile hepatocytes in pure resveratrol treated group show moderate histochemical reaction for glycogen accumulations (12-III) that became limited in optimized formula, F8, treated group where, accumulations present but in one pole of cell (12-IV). But in Reference treated group, minimal histochemical reaction for glycogen accumulations could be noticed (12-V).
The results obtained upon using PAC staining, were in agreement with that obtained from H&E and TB staining, which in turn augmented the biochemical results obtained.
Pharmacological study suggested that, the new optimized formula of resveratrol, F8, may exert a hepatoprotective effect higher than that obtained by pure resveratrol or the reference silymarin. Where, silymarin, as indicated by the histopathological studies, failed to improve the whole picture of the diseased liver tissues where, a marked degree of fibrosis, inflammation and steatosis are still observed in the liver tissues, compared to that treated with the optimized resveratrol formula, in which the liver tissues showed almost normal structure and restored its normal architecture.
Formulation of resveratrol as ternary complex with β-cyclodextrin and lecithin was an effective tool for enhancement of the solubility and hence the dissolution properties of resveratrol. Preparation of a ternary (resveratrol: β-cyclodextrin: lecithin) complex in the weight ratio 1: 2.5: 0.5 w/w by freeze drying technique (F8) showed better dissolution performance than that prepared by the solvent evaporation technique. The study revealed a profound hepato-therapeutic effect of ternary cyclodextrin complex (F8) which may serve as a promising alternative medicine.