ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Sham Kishor Kanneppady 1, Anusha Bhaskar 2, Prasanna Kumar Rao 3, Anusha Rangare Lakshman 4, Ankur Barua 5, Sowmya Sham Kanneppady 6
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Salivary analysis, a non-invasive alternative to serum analysis, is an effective modality for diagnosis and for prognosis prediction of various diseases like oral cancer and even oral potentially malignant disorders. The aim of this study was to evaluate the level of zinc (Zn) in saliva of patients with oral leukoplakia (OL) and to compare the values with healthy controls. The study group comprised of 25 clinically diagnosed and histopathologically confirmed cases of OL and 25 age- and sex-matched controls. The data were assessed statistically using SPSS version 17. Among 50% patients with OL, only 4% of patients showed speckled leukoplakia and the remaining 46% revealed homogeneous leukoplakia. The mean Zn level differed significantly (p<0.05) between the OL patients and the controls with patients exhibiting lower Zn (116.96±38.73) levels in contrast to controls who presented higher Zn (146.76±29.18). In conclusion, evaluation of salivary Zn may be used as a potential diagnostic tool in OL patients. Further research is needed in this line in patients with oral potential malignant disorders with higher sample size in order to correlate habit and diet pattern which may affect the Zn status.
Keywords |
| Oral leukoplakia, Zinc, Saliva, Oral potentially malignant disorders, Diagnostic tool. |
INTRODUCTION |
| Although rapid developments have been made in the field of medical science, cancer remains to be the principal cause for human death. It is estimated that more than one million new oral cancer cases are being identified annually in the Indian subcontinent, of which 90% are oral squamous cell carcinomas. It has been well established by researchers that virtually all oral cancer are preceded by visible clinical changes in the oral mucosa usually in the form of white or red patch [1]. Leukoplakia is defined as „„A white plaque of questionable risk having excluded (other) known diseases or disorders that carry no increased risk for cancer” [2]. The estimated prevalence of oral leukoplakia (OL), worldwide, is approximately two percent [3]. |
| The importance of zinc (Zn) in human nutrition and human health and disease has been extensively documented [4]. Zink influences many body system and function including growth, bone formation, brain development, reproduction, fetal development, sensory function (smell and taste), immune mechanism, membrane stability and wound healing [5]. Zink is present in many biological tissues and fluids such as plasma, serum, whole blood, erythrocyte, leukocyte, urine, hair and saliva [6, 7]. Many studies reported that trace elements like Zn play a major role as either inhibitory or causative agent of cancer. Several researchers studied the Zn levels in the serum, plasma and tissue of premalignant and malignant lesions of oral cavity and head and neck neoplasms. However, study using saliva samples were very few. Hence, the present study was undertaken to evaluate the Zn levels in the unstimulated whole saliva of patients with OL and age and sex matched controls. |
II. MATERIAL AND METHODS |
| Fifty patients between the age range of 20 and 65 years, reporting to the department of oral medicine and radiology in a dental college in south India were enrolled into the study. The study subjects included 25 histopathologically confirmed cases of OL and 25 age and sex matched healthy controls. A detailed case history with habit index was taken from each subject in the study. The Institutional Ethics Committee approval was obtained prior to start of the study and informed consent was taken from all the participants included in the study. All the subjects were explained about the objectives of the study. The descriptive data of total 50 patients was collected, evaluated and analyzed statistically using SPSS version 17 and probability value less than 0.05 was considered significant |
| Patients with systemic illness, longâÃâ¬Ãâterm drug intake, previous history of malignancy, or history of antioxidant medication were excluded from the study. Unstimulated saliva was collected from the study subjects between 9:00 am and 12:00 pm to avoid diurnal variation. The subjects were requested not to eat, drink, perform oral hygiene activities, or chew 60 min prior to the saliva collection procedure. The subjects were then seated on the dental chair and asked to spit in a graduated container every 1 min till 5 ml of saliva was obtained. During saliva collection, subjects were instructed not to speak or swallow. The salivary samples were stored at a temperature of -20°C. Prior to estimation, salivary samples were subjected for digestion by making use of concentrated nitric acid. The digested saliva samples were then read in the atomic absorption spectrophotometer. Means and standard deviations were calculated for salivary Zn in study and control subjects. Independent samples„tâÃâ¬ÃŸ test was used to compare the mean values of salivary Cu between the study subjects and control group. |
III. RESULTS |
| Among 50% patients with OL, only 4% of patients showed speckled leukoplakia and the remaining 46% revealed homogeneous leukoplakia (Table 1). |
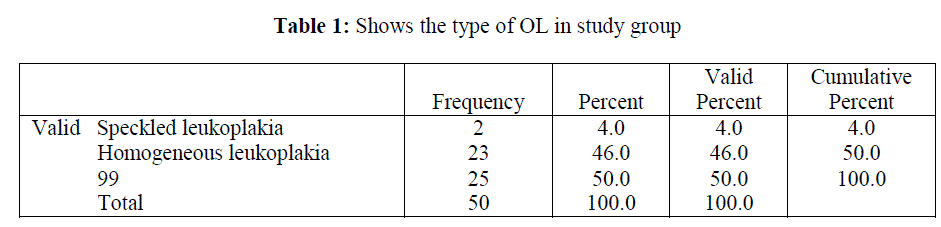 |
| The Zinc levels were measured in μg/dl. The mean Zn level of patients with OL was 116.96 with standard deviation being 38.73 and standard error mean 7.74. The mean Zn level in control subjects was 146.76 with standard deviation being 29.18 and standard error mean 5.83. The mean Zn level differed significantly (P=.003) between the OL patients and the controls with patients exhibiting lower Zn (116.96±38.73) levels in contrast to controls who presented higher Zn (146.76±29.18) (Table 2). |
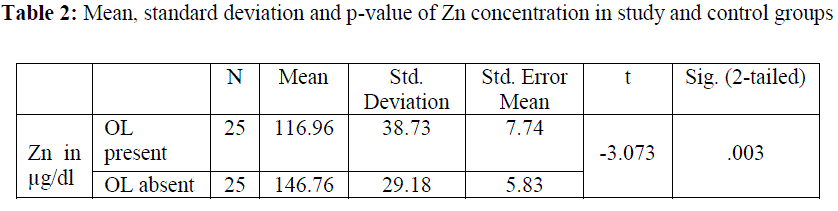 |
| Within the past few years, the use of saliva as a diagnostic tool has gained considerable attention and become a wellaccepted method. Salivary analysis, a non-invasive alternative to serum analysis, is an effective modality for diagnosis and for prognosis prediction of various diseases like oral cancer and even oral potentially malignant disorders. As a diagnostic fluid, saliva offers superiority over serum due to both a noninvasive collection method and a cost-effective approach for screening of large populations. Collection of saliva offers a reduced risk of infection compared to the collection of serum [8, 9]. |
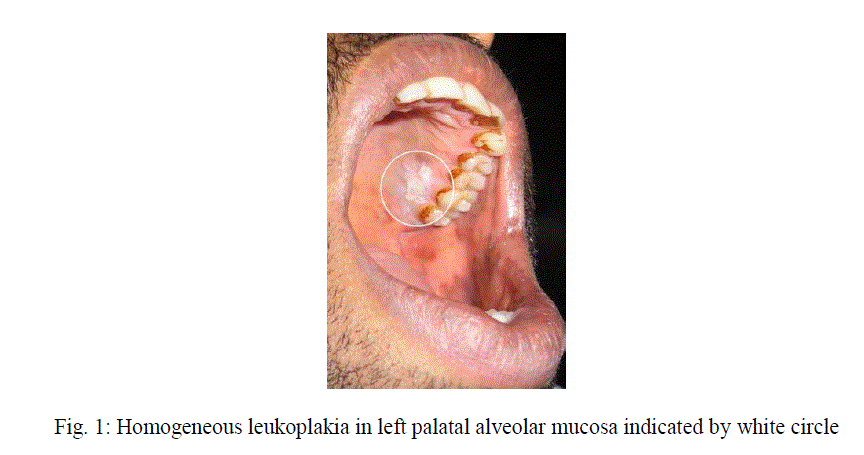
| Oral leukoplakia may affect any site of the oral and oropharyngeal cavity. Clinically OL can be subdivided into homogeneous type (flat, thin, uniform white in color) (Figure 1) and a non-homogeneous type. The non-homogeneous type has been defined as a white and red lesion („„erythroleukoplakia”), that may be either irregularly flat (speckled) or nodular. Verrucous leukoplakia is yet another type of non-homogeneous leukoplakia [10]. In our study most of the patientsâÃâ¬ÃŸ revealed homogeneous leukoplakia and very few cases showed specked leukoplakia. In a study from India, it was reported that annual malignant transformation rate of OL is 0.3% [11]. In studies from Western countries somewhat higher figures have been mentioned; an annual malignant transformation rate of approximately 1% is probably a reasonable average figure for all types of leukoplakia together. It is well appreciated that this figure is much higher for non-homogeneous types, [12] including proliferative verrucous leukoplakia. |
 |
| Trace elements play, directly or indirectly, an important role in various physiological metabolic processes in humans. Zinc is involved in carbonic acid (carbonic anhydrase), in proteolysis (carboxy peptidase, leucine amino peptidase, etc.) [13]. Bioelements e.g. Copper (Cu) and Zn are involved in vital biochemical activities like different redox and free radical formation and in maintaining cellular proton homeostasis [14]. Zinc is essential for regulation of cell cycle and cell division and also essential for DNA polymerase activity and is particularly important for rapid cell proliferation encountered in growing tumors. A study comparing the Cu and Zn levels in saliva of patients with oral cancer to the control group highlighted that these elements were significantly higher in the case group when compared to controls. The same researchers also have done a similar study in the serum and found that Cu and Zn levels were higher in case group when compared to control group [15]. An another study of serum Cu and Zn levels in submucous fibrosis and leukoplakia showed decreased levels of serum Cu and Zn in submucous fibrosis and there was no change in leukoplakia when compared to controls [16]. There are few studies reported in the literature [17-19] where low levels of serum Zn were found in oral cancer patients compared to controls. In our study also we observed a significant decrease in salivary Zn level in OL patients when compared to controls. The reduction in salivary Zn in OL patients may be explained on the basis that dysplastic cells and tissue have increased metabolic requirement of Zn which result in an increased uptake from adjacent structure such as salivary secretion. |
V. CONCLUSION |
| Evaluation of salivary Zn may be used as a potential diagnostic tool in OL patients. Further research is needed in this line in patients with oral potential malignant disorders with higher sample size in order to correlate habit and diet pattern in these patients which may affect the Zn status. |
References |
|