ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Vilas Gade1, Pankaj Nilkanth1, Ganesh Mhaske1, Rajesh Manjul1, Vikki Shinde1, Sachin Kushare1, Sharad Shelke1*
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
In present investigation tetramethylguanidine based co-polymeric gel was prepared and characterized by I.R. spectroscopy. This gel was used as catalyst in synthesis of various formanilide and thiocarbamate derivatives. Among synthesized compounds representative compounds were characterized by spectral studies. Such co-polymer gel is reused for several cycles with constant efficiency
Keywords |
| Acryl amide, 1,1,3,3-tetra methylguanidine acrylate, Bisacrylamide, Co-polymer gel |
INTRODUCTION |
| For the perspective of organic chemist, the relevance of polymer has changed and evolved dramatically over past half century. From their early use in peptide and oligosaccharide synthesis1 to more recent preparation of small organic molecule libraries2, polymers have been used to aid in reaction manipulation and product isolation. Polyfunctionalized polymers have recently gained great practical importance because of their industrial applications3,4. Non-conventional catalyst like polystyrene is noncorrosive and easy to separate from reaction mixture. They can also be used repeatedly over prolonged period without any difficulty in their handling and storage5. Some polymer catalysts are applicable for various organic conversions6. Polymers with attached transition-metal complexes have recently been developed to catalyze hydrogenation, hydroformylation of olefins and carbonylation of methanol7. |
| One of the most useful and versatile functional groups to be introduced in an organic molecule is formyl group. Formamides are widely used in organic synthesis as protecting group of amines8, precursor for isocyanide preparation9. In addition they have been used in synthesis of formamidines10. Acetic formic anhydride11 continues to be most widely used formylating reagent but it is sensitive to atmospheric moisture. Recently polyethylene glycol has been reported as medium for formylation of aniline derivatives with formic acid12. |
| Esters belong to wide range of category ranging from aliphatic to aromatic with various substitutions and multifunctional groups13. Esterification is industrially important reaction producing organic esters at the level of tones per year. Various ionic liquids were immobilized on polystyrene using different coupling agents and showed high activities for expeditious synthesis of homoallylic alcohols and esterification14. |
| Due to variety of applicability of co-polymer gel in the field of organic synthesis, we prompted us to study the catalytic usabilities of co-polymer gel. |
II .RELATED WORK |
| Literature survey reveals that various methods have been reported for N-formylation of amines. Acetic formic anhydride, chloral15, ammonium formate 16, aq. 85% formic acid & ZnO17, activated formic acid using DCC9 or EDCI18. However these methods have several drawbacks like delayed reaction times, elevated temperature, use of toxic formylating agents & catalyst. Use of guanidine based ionic liquids as a catalyst for N-formylation of amines is also reported in the literature19. Synthesis of thiocarbamates by alcoholysis of isothiocyanates is also known in literature20. |
III. RESULT AND DISCUSSION |
| In present investigation preparation of co-polymer gel catalyst is outlined in scheme 1. The co-polymer catalyst is prepared from three monomers AM, GACL & BAM. The synthesized co-polymer catalyst has been characterized by I.R. spectroscopy. |
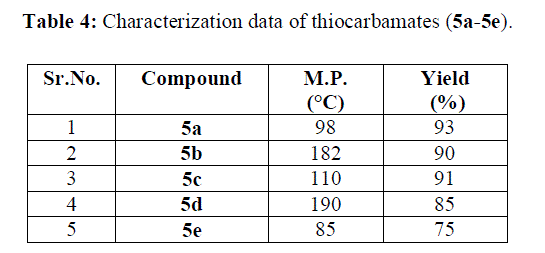
| The synthetic route used for preparation of various formanilides and thiocarbamates is outlined in scheme 2. The copolymer gel is used as catalyst in the synthesis of various formanilide derivatives from substituted anilines and formic acid and also in the synthesis of various thiocarbamates from isothiocyanates of corresponding anilines and ethanol. The structure of synthesized compounds has been confirmed by spectral studies. |
| Scheme 1 |
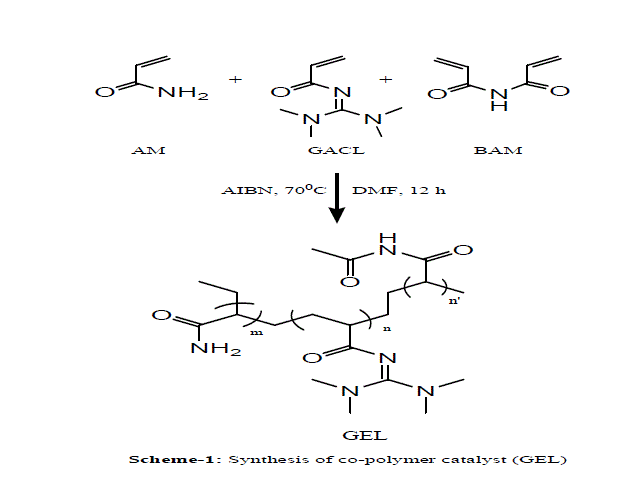 |
| Scheme 2 |
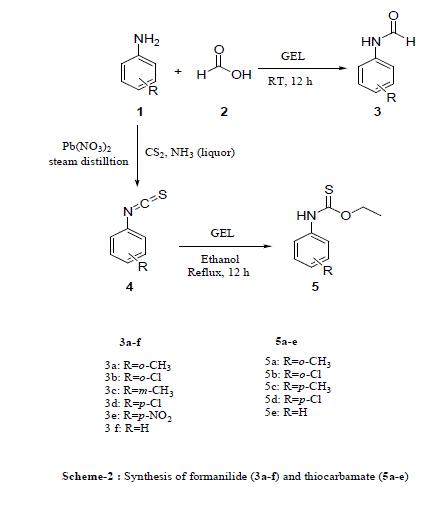 |
IV. CONCLUSION |
| The organopolymer gel was successfully prepared and it is stable to air. GEL catalyst has been showed that it is good to use for multicomponent reaction with 70 to 80% yield. The catalytic activity of GEL remains unaltered making them environmentally benign and with reusability due to their efficiency, ease of handling and cost effectiveness. We believe that this approach towards sustainable chemistry has great scope for future catalytic process. |
V. EXPERIMENTAL |
| All melting points were recorded in an open capillary tube in liquid paraffin bath and are uncorrected. The purity and progress of the reaction was routinely monitored by thin layer chromatography. I.R. spectra were recorded on SHIMADZU FTIR spectrophotometer on KBr disc. 1H NMR spectra were recorded on BRUKER- AVANCE 400 MHz spectrometer with peak values shown in δ ppm using TMS as internal standard when measured in DMSO-d6. Signal multiplicities are shown by s (singlet), d (doublet), t (triplet), q (quartet) and m (multiplet). Substituted anilines and required chemicals for the preparation of precursors were purchased from commercial chemical supplier. TLC was performed on pre-coated silica gel aluminium plates (Kieselgel 60, 254, E. Merck, Germany) |
| General procedure for the preparation of GEL |
| In a 50 ml two neck R.B.F. equipped with a magnetic stirring bar and nitrogen inlet were charged AM (0.01 mole), GACL (0.01 mole), BAM (0.01 mole) and AIBN (Catalytic) in DMF (50 mL). The reaction mixture was carefully degassed by bubbling nitrogen in reaction mixture and then heated at 70°C under nitrogen atmosphere for 12 hours. Then the reaction mixture was concentrated and precipitated in to excess pet ether. The obtained co-polymer was separated and dried in vacuum oven at RT. |
| FT-IR (KBr) v/max (cm-1): 3184, 2949, 1668, 1602, 1290, 1232. |
| General procedure for the preparation of formanilide derivatives (3a-f) A 50 ml R.B.F. was charged with substituted aniline (0.01 mole), formic acid (0.01 mole) and GEL (0.359 gm). Then reaction was stirred for 12 hours at RT. The progress of the reaction was monitored by TLC. After completion, product was separated by filtration. The recovered catalyst was used for further reactions. |
| N-o-tolylformamide (3a); FT-IR (KBr) v/max (cm-1): 2879.82, 1689.70, 1660.77, 1456; 1H NMR (400 MHz, DMSO-d6): 2.49 (3H, s), 4.78 (1H, s, -NH), 7.08 (2H, t, J = 7.3 Hz), 7.29 (2H, t, J = 7.6 Hz), 9.1 (1H, s). |
| General procedure for the preparation of thiocarbamate derivatives (5a-e) A 50 ml R.B.F. equipped with reflux condenser was charged with substituted phenyl isothiocyanate (0.01 mole), ethanol (5 mL) and GEL (0.359 gm). Then reaction was refluxed for 12 hours. The progress of the reaction was monitored by TLC. After completion of reaction, product was separated by filtration. The recovered catalyst was further used for next cycle. |
| O-ethyl N-4-chlorophenylcarbamothioate (5d); FT-IR (KBr) v/max (cm-1): 2989.76, 1683.77, 1423, 1278.85, 613.38; 1H NMR (400 MHz, DMSO-d6): 0.87 (3H, t, J = 6.6 Hz), 3.9 (2H, q, J = 6.7 Hz), 4.4 (1H, s, -NH), 7.08 (2H, m, ArH), 7.45 (1H, d, J = 8 Hz), 7.71 (1H, d, J = 7.7 Hz). |
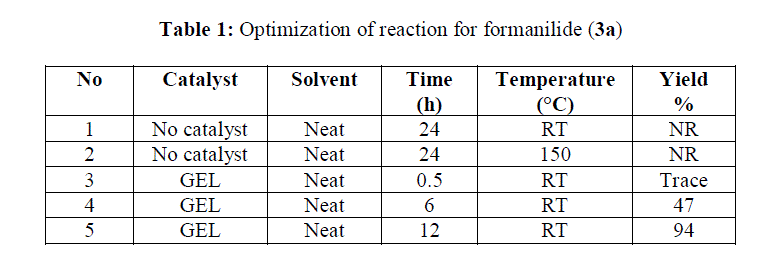 |
| Reaction without catalyst at room temperature and 150°C for 24 hours showed no product formation. By using co-polymer gel as a catalyst, reaction goes to completion in 12 hours at RT with 94% isolated yield. |
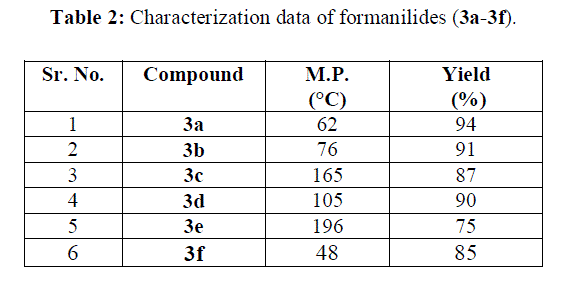 |
| In view of the results shown in table 1, various substituted anilines were subjected for formylation in presence of copolymer gel as catalyst and isolated yields were found in between 75-94% |
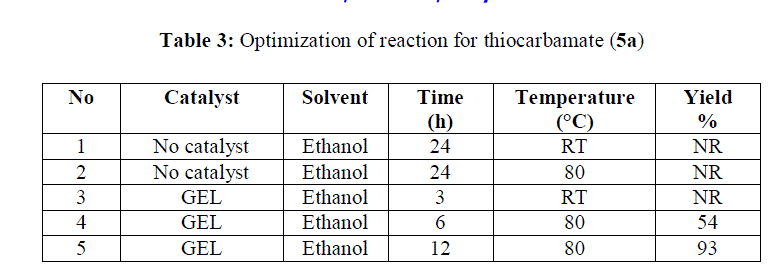 |
| Reaction without catalyst in ethanol at RT and 80°C for 24 hours not showed the formation of product. Consumption of starting material was complete when reaction was carried out by using co-polymer gel catalyst in ethanol at 80°C for 12 hours. |
 |
| As per the results of various reaction conditions employed as shown in table 3, various isothiocyanates are subjected for preparation of thiocarbamates and the isolated yields were found in between 75-93% |
VI. ABBREVATIONS |
| AM:- Acryl amide, GACL:- 1,1,3,3-tetra methylguanidine acrylate, BAM:- Bisacrylamide, GEL:- Co-polymer gel, RT: - Room Temperature, NR: - No Reaction |
ACKNOWLEDGEMENTS |
| The authors are thankful to Principal and Dr. A. B. Nikumbh, Head, Dept. of Chemistry, S.S.G.M. College, Kopargaon, Ahmednagar (MS) for providing necessary research facilities and constant encouragement |
References |
|