ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
M.Dhananjayulu1, M.Siva Prasad1, Ch.Swarupa1, M.Seenu naik1 and N.Y.Sreedhar2*
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
A novel method for the synthesis of 2-Hydroxy chalcones via Claisen-Schmidt is introduced using Silicagel supported piperidine as an alternative base. The reaction is clean with excellent yield, shorter reaction time and reduces the use of volatile organic compounds (VOCs) and Its electrochemical reduction behaviour explained. The structures of the synthesized compounds were confirmed by IR, mass spectroscopy and elemental analysis.
Keywords |
| Chalcone, Claisen-Schmdit condensation, Silicagel supported piperidine, IR, Mass and Elemental spectral analysis. |
INTRODUCTION |
| Chalcone is an aromatic ketone that forms the central core for a variety of important biological compounds, which are known col- lectively as chalconoids. They show antibacterial, antifungal, antitumor and anti-inflammatory properties [1]. Some cha- lcones demonstrated the ability to block voltage-dependent potassium channels [2]. They are also intermediates in the biosynthesis of flavonoids, which are substances wide- spread in plants and with an array of biological activities. Chalcones are also intermediates in the Auwers synthesis of flavones. Due to ineffective drugs, cancer is the second most leading cause of death after heart attack. Therefore, the researchers have accelerated their efforts for the gen- eration of new anticancer drugs with high therapeutic in- dex. In this connection, a clinically effective antitumor derivative of chalcone (calicheamicin) has stimulated the investigators to concentrate their studies on chalcones and related compounds [3]. |
| Chalcones, 1,3-diphenylpropenones constitute one of the major classes of flavonoids with widespread distribution in vegetables, fruits, tea and soy.[3, 4] Prehistoric therapeutic applications of chalcones can be associated with the thousandyear old use of plants and herbs for the treatment of different medical disorders. [5] Contemporary studies report a generous variation of significant pharmacological activities of chalcones including antiproliferative, antioxidant, anti-inflammatory and anticancer effects.[4, 6-8 ] |
| Chalcones are important precursors in the biosynthesis of flavones and flavanones and are usually synthesized from acetophenones and benzaldehydes via the Claisen-Schmidt condensation, using base in a polar solvent. [9-11]. In addition, more exotic synthetic protocols have been reported, such as the palladium-mediated Suzuki coupling between cinnamoyl chloride and phenyl boronic acids or the carbonylative Heck coupling with aryl halides and styrenes in the presence of carbon monoxide.[12, 13 ] |
| Electrochemical methods are very sensitive for the determination of organic molecules, including drugs, pesticides and related molecules in pharmaceutical dosage forms and biological fluids [14-15]. The electrochemical techniques are soo advanced in the field of analysis of drugs is due to their simplicity, low cost and relatively short analysis time when compared to the other techniques. The use of carbon based electrodes, especially glassy carbon electrode, for electroanalytical measurements has increased in recent years because of their applicability to the determination of substances that undergo redox reactions, a matter of great importance in the field of clinical and pharmaceutical analysis. Redox properties of drugs can give insights into its metabolic fate or their in vivo redox processes or pharmaceutical activity [16,17]. |
EXPERIMENTAL |
| All the aldehydes and ketones were purchased from S.D. fine Chemicals (India). and silicagel supported piperidine was purchased from sigma aldrich. Melting points were uncorrected and determined in an open capillary tube. IR spectra were recorded in KBr on al JASCO FT / IR- 5300. The mass spectra were recorded on LCMS-2010 DATA REPORT SHIMADZU. Elemental analysis was carried out on a Flash Ea 1112 Series Chn Report Thermo Finnigan. Chalcones were synthesized by clasien- Schmidt condensation using silica gel supported piperdine. The chemicals and solvents used were of laboratory grade and were purified completion of the reaction was monitored by thin layer chromatography on precoated sheets of silica gel-G (Merck, Germany) using iodine vapour for detection. |
| Voltametric experiments were conducted with Autolab PGSTAT 101 supplied by Metrohm, The Netherlands. All electrochemical measurements were performed using an electrochemical workstation, having a conventional three-electrode cell configuration with a GCE of a diameter of 3 mm as the working electrode, saturated calomel electrode (SCE) as a reference electrode and platinum wire as a counter electrode. Electrochemical experiments were carried out in a voltammetric cell at room temperature (25oC). All potentials are referred to the Ag/AgCl reference electrode. All potentials are quoted vs. SCE reference electrode. All pH measurements were made with the aid of a digital pH meter using a combined glass electrode. |
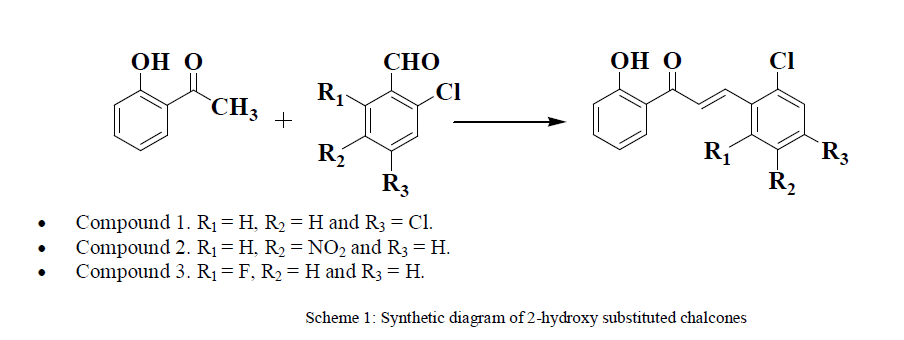
GENERAL METHOD FOR SYNTHESIS OF CHALCONES |
| An equimolar mixture of 2-hydroxy acetophenone, aromatic aldehydes and Silicagel supported piperdine (2mmol) was stirred in methanol (15 ml) at 40°C for 1 hour. After the completion of the reaction (monitored by TLC), the crude mixture was worked up in ice-cold water (100 ml). The product which separated out was recrystalised from methanol. The filtrate was evaporated to remove water leaving Silicagel supported piperdine behind. The same was utilized to synthesize further chalcone as well as to use as electrolyte in voltammetric technique. |
 |
SYNTHESIS AND CHARACTERIZATION |
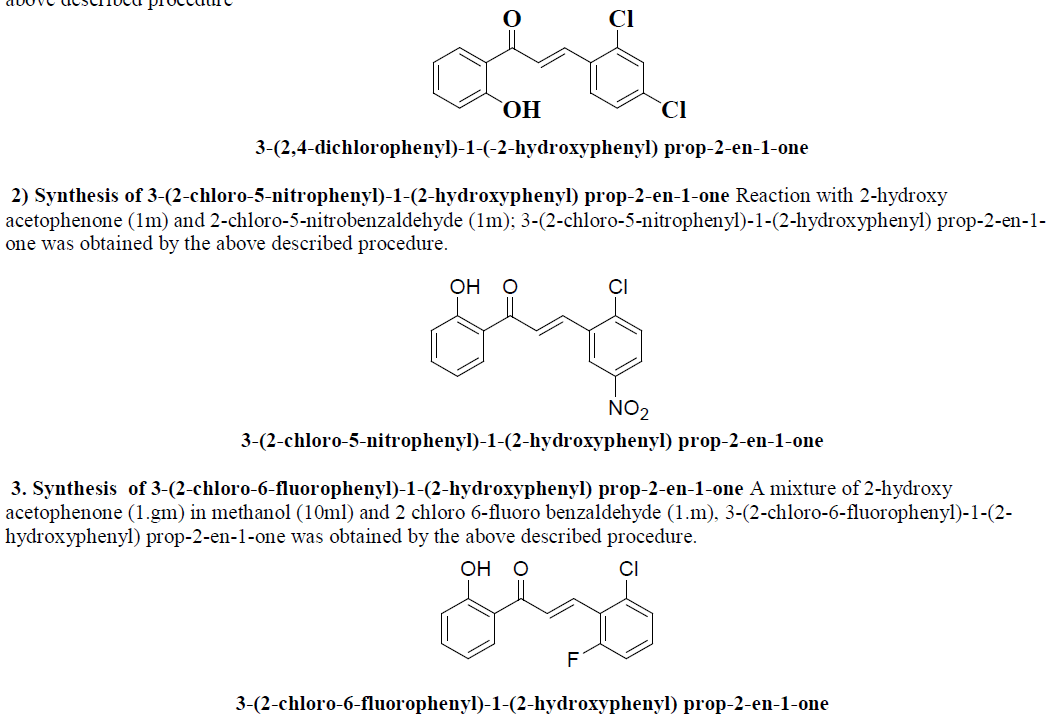
| 1) Synthesis of 3-(2,4-dichlorophenyl)-1-(-2-hydroxyphenyl) prop-2-en-1-one An equimolar mixture of 2-hydroxy acetophenone, 2,4 dichlorobenzaldehyde; 3-(2,4-chlorophenyl)-1-(-2-hydroxyphenyl) prop-2-en-1-one was obtained by the above described procedure |
 |
RESULTS AND DISCUSSION |
| Literature indicates hydroxyl substitutions on benzyl rings greatly affected the reactivity. The hydroxyl-substituted (phenolic hydroxyls) benzaldehydes and acetophenones are easy to ionize in NaOH solution and the carbonyl-C seemed to fail to retain its original polarity, the reaction would fail [16,17], In this study, we found piperidine catalysis seems a highly effective method in Claisen-Schmidt condensation for synthesizing hydroxy-substituted chalcones. Piperidine is successfully used as a catalyst in the syntheses of above proposed compounds. |
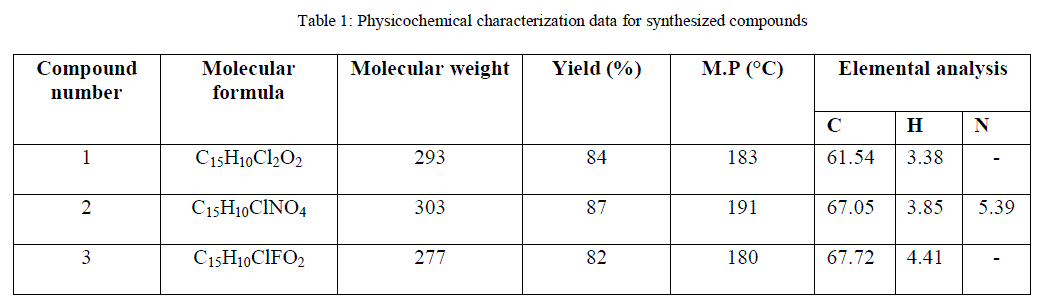
| In the claisen-schemidt condensation of chalcones synthesis, 2’ – hydroxy functional group may cyclise to the corresponding flavanones under higher concentration of alkali, also, side reactions such as multiple condensation polymerizations and rearrangements are common, these undesirable side reaction decreases the yields of the target adduct and render their purification difficult [18] so, it was planned to use a weaker base like piperidene instead of using strong baseto get the better yields. In present paper, we report piperidine mediated synthesis of hydroxy chalcones for getting better results. The structures of the compounds have been established on the basis of elemental (C, H, and O) analysis, IR, 1H NMR, MS spectral data and their electrochemical behavior was screened out using cyclic voltametry. The spectral characterization and the obtained results are given in following Table. |
 |
 |
CYCLICVOLTAMETRY STUDY |
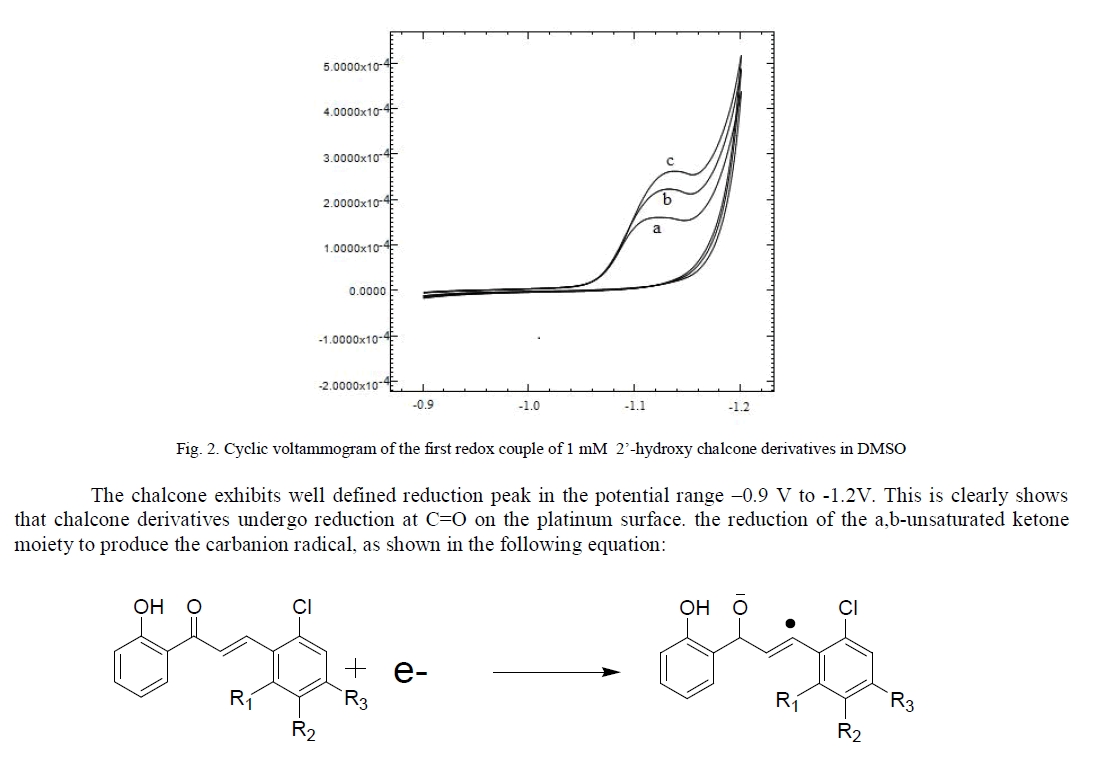
| The reduction of chalcones involved that the first electron attack takes place on the carbonyl group and the free radical formed undergoes isomerisation into another free radical which is further reduced or can be converted into a dimer [19, 20].we have used cyclic voltammetry in order to quantitatively characterize the formation and stability of the anion radical from three synthesized 2’- hydroxy chalcones. All the three synthesized 2’-hydroxy chalcones were electrochemically reduced on platinum electrode in a acetonitrile medium containing and silicagel supported piperdine which used as catalyst in synthesis as well as supporting electrolyte electrolyte. The reduction pattern of the three compounds was similar. (Fig. 2). |
 |
ACKNOWLEDGEMENT |
| I am very much thankful to UGC-BSR for providing financial assistance. |
References |
|