ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Sangappa K Ganiger1, Chaluvaraju B V2, Murugendrappa M V3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
In-situ polymerization of pyrrole (Py) was carried out with strontium arsenate(ceramic)in the presence of oxidizing agent ammonium persulphate to synthesize polypyrrole (PPy)/ strontium arsenate[Sr3(AsO4)2]by chemical oxidation method. The PPy/Sr3(AsO4)2composites were synthesized with various compositions viz., 10, 20, 30, 40 and 50 wt. % ofSr3(AsO4)2 in Py. The surface morphologies of these composites were analyzed using Scanning Electron Microscopy (SEM) show that Sr3(AsO4)2 particles were embedded in PPy chain to form multiple phases. The Fourier Transform Infra-Red Spectroscopy (FTIR) reveals, the stretching frequencies were shifted towards higher frequency side. The powder X-ray diffraction (XRD) spectrograph suggests that, they exhibit semi-crystalline behavior. Thermograph of thermal analysis (TG/DTA) were shown that, the PPy/Sr3(AsO4)2composites have stronger stability than PPy. The frequency dependent A.C. conductivity reveals that, the Sr3(AsO4)2 concentration in PPy is responsible for the variation of conductivity of the composites. The value of the conductivity was increases for wt. 20% Sr3(AsO4)2in PPy. The dimensions of Sr3(AsO4)2 particles in the matrix have influence on the conductivity values.
Keywords |
| Polypyrrole; Strontium arsenate; Composites; Conductivity; Frequency |
INTRODUCTION |
| During the last 30 years, however, conducting polymers characterized by better electrical conductivities have been found.These materials have attracted the interest of both academic and industrial researchers in fields ranging from Chemistry to Solid State Physics and Electrochemistry. The close interaction between scientists from diverse background has been major factor in the rapid development in the field of conducting polymers. The discovery of electrical conductivity in molecular charge transfer promoted the development of conducting polymers which have been synthesized and show the excellent electrical properties. Conducting polymers, by virtue of their light weight and greater ease of fabrication, have replaced and are continuing to replace metals in several areas of applications. Conducting polymers have been prepared for a wide range of applications ranging from rechargeable batteries to smart windows. |
| Polypyrrole (PPy) has become one of the most studied electronically conducting polymers. It can be synthesized either chemically or electro-chemically. Polypyrrole is an intrinsic conducting polymer which can be made to have conductivities up to 1000 S cm–1 rendering its versatile applications in batteries, electronic devices, functional electrodes, electro-chromic devices, optical switching devices, sensors and so on [1–5]. |
II. LITERATURE REVIEW |
| Researchers have reviewed recent research work on polypyrrole type conducting polymers. Research and development work had been reviewed with special interest in chemical, electrochemical, doping process and polypyrrole based composites processes. Process development with high accuracy of product results had the direct relationship with structure and properties. According to them, polypyrrole based on a variety of structures generated from molecular structural modification could give more interesting results for future applications. |
| Combining two kinds of materials to form composite may lead to a synergy effect and help to enhance each other, which are helpful for improving sensor properties such as thermal stability, lower operating temperature and fast response and recovery. Conducting polymer/metal oxide nano-composites have been synthesized in most recent times and based on these nano-composites gas sensors, humidity sensors and biosensors have also been developed by few research groups. However, the conducting polymer/metal oxide based sensors are still under development and new concepts are continuously tested all over the world. |
| This study presents the design and fabrication of a capacitive micro humidity sensor integrated with a fivestage ring oscillator circuit on chip using the complementary metal oxide semiconductor (CMOS) process. The area of the humidity sensor chip is about 1 mm2. The humidity sensor consists of a sensing capacitor and a sensing film. The sensing capacitor is constructed from spiral inter-digital electrodes that can enhance the sensitivity of the sensor. The sensing film of the sensor is polypyrrole, which is prepared by the chemical polymerization method, and the film has a porous structure. The sensor needs a post-CMOS process to coat the sensing film. The post-CMOS process uses a wet etching to etch the sacrificial layers, and then the polypyrrole is coated on the sensing capacitor. The sensor generates a change in capacitance when the sensing film absorbs or desorbs vapour. The ring oscillator circuit converts the capacitance variation of the sensor into the oscillation frequency output. Experimental results show that the sensitivity of the humidity sensor is about 99 kHz/%RH at 25 °C. |
III. EXPERIMENTAL DETAILS |
| SYNTHESIS |
| The AR grade [SpectroChem Pvt. Ltd.] pyrrole [6] was purified by distillation under reduced pressure. 0.3 M pyrrole solution was contained in a beaker which was placed in an ice tray mounted on a magnetic stirrer. 0.06 M ammonium persulphate [7] solution was continuously added drop-wise with the help of a burette to the above 0.3 M pyrrole solution. The reaction was allowed for 5 hours under continuous stirring by maintaining a temperature of 0 °C to 5 °C. The precipitated polypyrrole was filtered and dried in hot air oven and subsequently in a muffle furnace at 100 °C. The yield of the polypyrrole was 3.6 g which has taken as 100 wt. %. |
| For 0.3 M pyrrole solution, 0.36 g (wt. 10%) of Strontium arsenate[Sr3(AsO4)2] was added and mixed thoroughly. Further 0.06 M ammonium persulphate was continuously added drop-wise with the help of a burette to the above solution to get PPy/Sr3(AsO4)2(wt. 10%) composite. Similarly, for 20, 30, 40 and 50 wt. %, 0.72 g, 1.08 g, 1.44 g and 1.8 g of Sr3(AsO4)2[Sisco Research Lab Ltd.] powder [8] is taken and the above procedure is followed to get PPy/ Sr3(AsO4)2 composites. The pure PPy and PPy/Sr3(AsO4)2 powder was pressed in the form of pellets of 1 cm diameter using hydraulic press. The conducting silver paste was applied to the pellets of synthesized composites to act as electrodes. The A.C. conductivity of the synthesized composites was measured in the frequency range from 101 Hz to 107 Hz. |
| CHARACTERIZATION |
| The SEM [6-12] images of the pure PPy, PPy/Sr3(AsO4)2 (wt. 50%) composite and Sr3(AsO4)2 were recorded using Scanning Electron Microscope (Jeol 6390LV). The FTIR [6-7, 10-12] spectra of were recorded on FTIR (Thermo Nicolet Avatar 370) spectrometer in KBr medium at room temperature. The XRD patterns were recorded on X-ray Diffractometer (Bruker AXS D8 Advance) [6- – 80°. Thermal analysis studies/testing were done in the heat range from 40 °C to 740 °C at 10 °C/min for the pure PPy, PPY/Sr3(AsO4)2 (wt. 50%) composite and Sr3(AsO4)2 using Thermal Analysis System (TG/DTA) (Perkin Elmer Diamond TG/DTA). |
III.RESULT AND DISCUSSION |
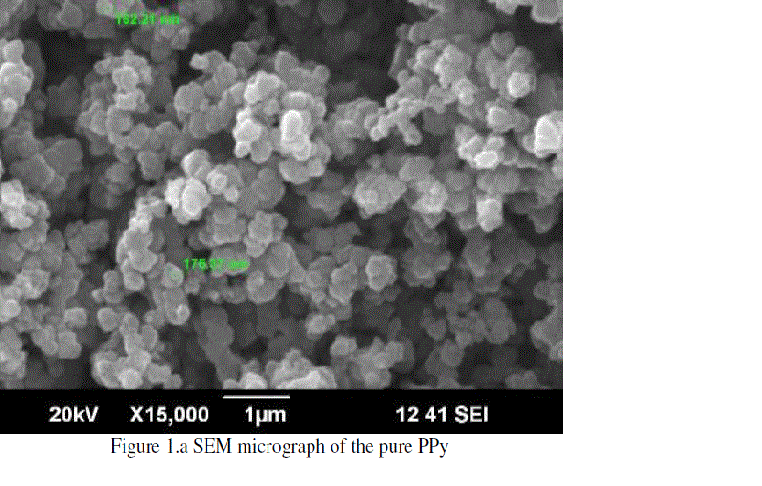 |
| The Figure 1.a represents the SEM micrograph of pure PPy. The figure represents the size and spherical nature of PPy particles. The SEM micrograph is shown a characteristic peak of amorphous polypyrrole. The elongated chain pattern of the polypyrrole particles was observed. Two particles sizes were measured as 162.21 nm and 176.37 nm. |
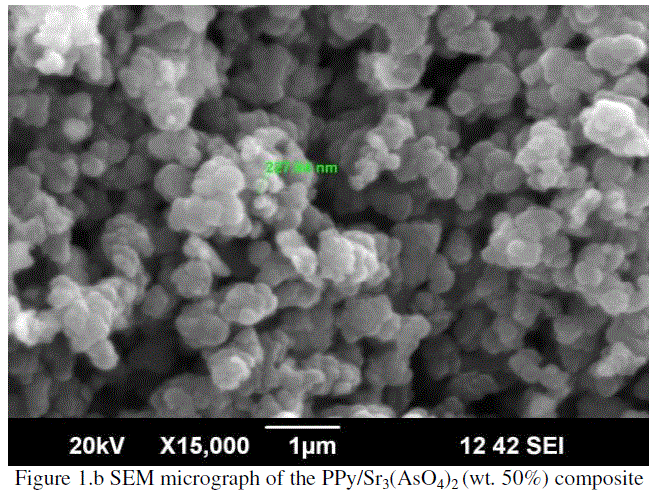 |
| The Figure 1.b represents the SEM micrograph of the PPy/Sr3(AsO4)2 (wt. 50%) composite and shown semicrystalline nature. Here, particle size was increased and measured as 227.84 nm. These show that, the Sr3(AsO4)2 particles were embedded uniformly in PPy chain to form multiple phases, presumably because of weak inter-particle interactions. |
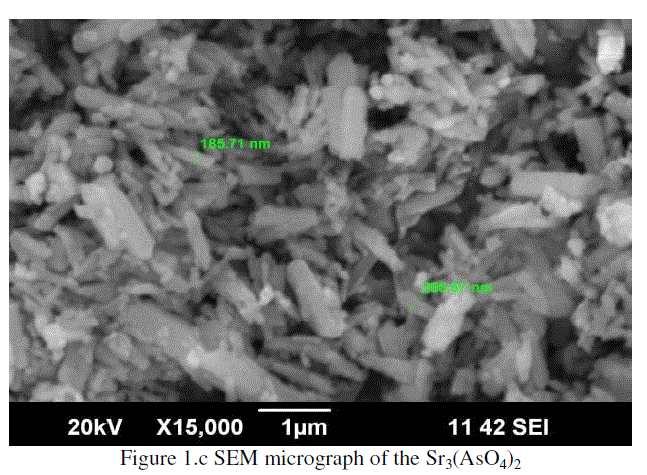 |
| The SEM micrograph of Sr3(AsO4)2 is shown in Figure 1.c and Sr3(AsO4)2 has semi-crystalline nature [6-12]. |
| FTIR Analysis |
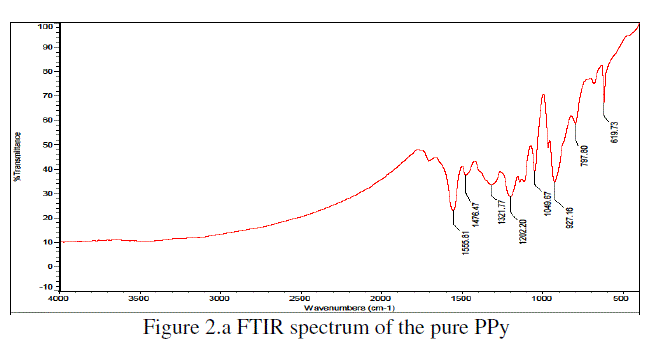 |
| The Figure 2.a is shown the FTIR spectra of pure PPy. The characteristic frequencies were observed at 1555 cm-1, 1476 cm-1, 1321 cm-1, 1202 cm-1, 1049 cm-1, 927 cm-1, 797 cm-1 and 619 cm-1 for pure PPy. The Figure 2.b is shown the FTIR spectra of PPy/Sr3(AsO4)2 (wt. 50%) composite.The characteristic frequencies were observed at 1560 cm-1, 1478 cm-1, 1321 cm-1, 1196 cm-1, 1049 cm-1, 927 cm-1, 798 cm-1, 684 cm-1& 619 cm-1 for PPy/Sr3(AsO4)2 (wt. 50%) composite. |
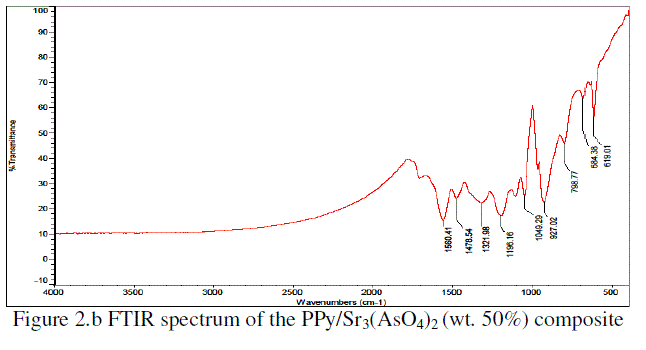 |
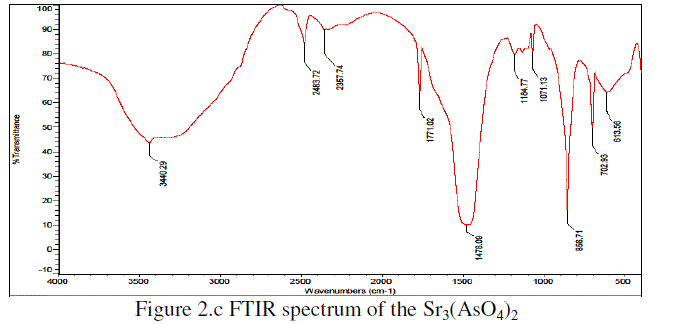
| The Figure2.c is shown the FTIR spectra of Sr3(AsO4)2.The characteristic frequencies were observed at 3040, 2483, 2357, 1771, 1478 cm-1, 1184 cm-1, 1071 cm-1, 856 cm-1, 702 cm-1& 613 cm-1 for Sr3(AsO4)2 respectively. |
| The stretching frequenciesmay be attributed due to the presence of C=N stretching, N–H bending deformation, C–N stretching and C–H bending deformation frequencies. The stretching frequencies were shifted towards higher frequency side when pure PPy was compared with PPy/Sr3(AsO4)2 (wt. 50%) composite. This indicates that, there is homogeneous distribution of Sr3(AsO4)2 particles in the polymeric chain due to the Van der Walls interaction between polypyrrole chain and Sr3(AsO4)2 [6-7, 10-14]. |
 |
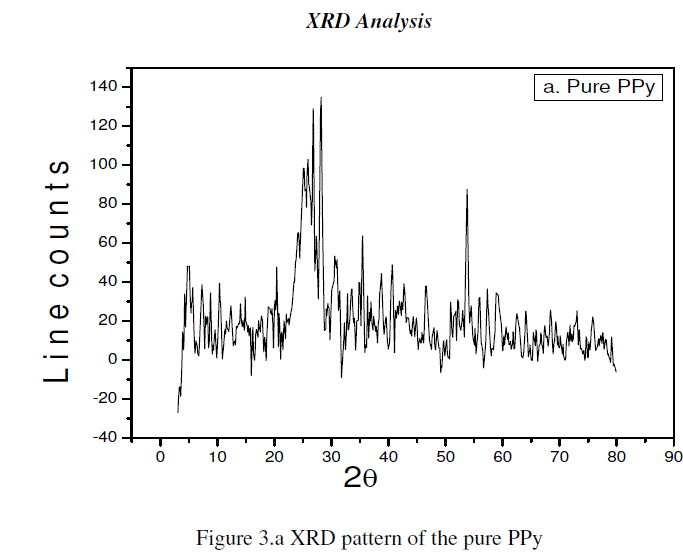
| The Figure 3.a represents the XRD pattern of pure PPy. This has a broad peak at about 2θ=25°, shown a characteristic peak of amorphous PPy. |
 |
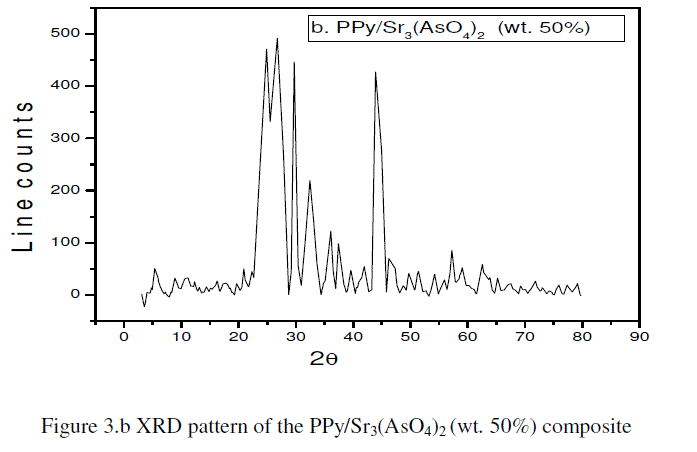
| The XRD pattern of PPy/Sr3(AsO4)2 (wt. 50%) composite shown in the Figure 3.b. The characteristic peaks were indexed by lattice parameter values. The main peaks were observed with 2θ at 20.9°, 23.3°, 24.8°, 26.72°, 29.7°, 32.4°, 36°, 43.9°, 57.2° and 62.5° with respect to inter-planar spacing (d) 4.2 Å, 3.8 Å, 3.5 Å, 3.32 Å, 3 Å, 2.7 Å, 2.4 Å, 2 Å, 1.6 Å, and 1.4 Å respectively. Careful analysis of the XRD of the PPy/Sr3(AsO4)2 (wt. 50%) composite suggests that, it exhibits semi-crystalline behaviour. |
 |
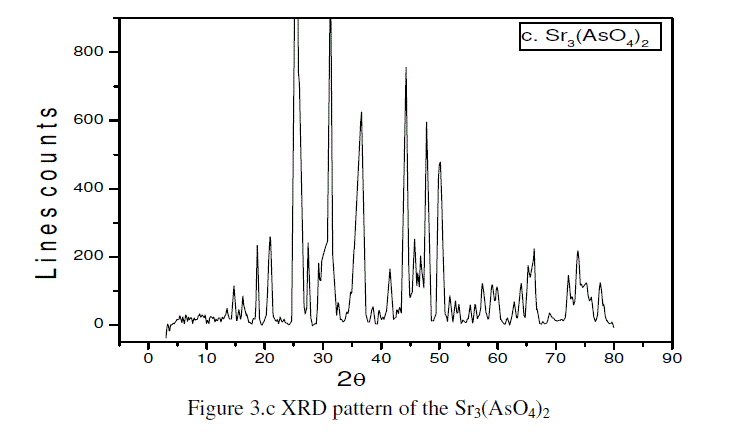
| The Figure 3.c represents the XRD pattern of the strontium arsenate [Sr3(AsO4)2 ]revealing the semicrystalline nature [6-11]. |
| TG/DTA Analysis |
| The most important and reliable factor in the study of heat stable polymers is the measurement or evaluation of thermal stability. Thermal properties and interaction between the polymers can also be noted from the oxidative degradation curves through thermo-gravimetric analysis (TG/DTA) studies. DTA is most commonly used to determine transition temperatures such as glass transitions, melting cross-linking reactions and decomposition. However, it measures only the total heat flow and the sum of all thermal transitions in the sample. |
 |
| The representative TG/DTA curves for pure PPy, PPy/Sr3(AsO4)2 (wt. 50%) composite and Sr3(AsO4)2 are shown in Figures 4.a-4.c respectively. The samples were heated from 40 °C to 740 °C under a constant heating rate of 10 °C/min and in the inert atmosphere of nitrogen gas. The residual weights (γc) of the pure PPy, PPy/Sr3(AsO4)2 (wt. 50%) composite and Sr3(AsO4)2 were reported at 736.3 °C, 734.7 °C and 735.1 °C respectively. Variation of weight loss is almost linear and the maximum polymer decomposition temperature is there from 40 °C to 740 °C for all. In the Figure 4.a, two major weight loss stages for PPy were observed at 110 °C to 130°Cand 736.3 °C. In the Figure 4.b, two major weight loss stages for PPy/Sr3(AsO4)2 were observed at 120 °C to 140 °C, 200 °Cand 734.8 °C. And in the Figure 4.c, three major weight loss stages for PPy/Sr3(AsO4)2 were observed at 120 °C to 140 °C, 625.26 °C and 734.5 °C. |
 |
| Derivative weight (mg/min) versus temperature is shown in Figures 5.a-5.c for the pure PPy, PPy/Sr3(AsO4)2 (wt. 50%) composite and Sr3(AsO4)2 respectively. For pure PPy, 0.052 mg/min is decomposed at 64.97 °C mg/min and 0.064 mg/min is decomposed at 240.54 °C with respect to total weight of the sample i.e. 4.057 mg. For PPy/Sr3(AsO4)2 (wt. 50%) composite, 0.044 mg/min is decomposed at 255.51 °C mg/min with respect to total weight of the sample i.e. 4.272 mg. It is found that, the weight loss caused by the volatilization of the small molecules in PPy/Sr3(AsO4)2 (wt. 50%) composite at different temperatures is slow compared to that of pure PPy and indicates its higher stability, which clearly proves that Sr3(AsO4)2 was inserted into the PPy to form composite and has increased the thermal stability of the composite material [15-23]. |
 |
| Figure 6.a, 6.b and 6.c Variation of A.C. conductivity is as a function of frequency for pure PPy, PPy/ Sr3(AsO4)2 [wt. 20% & wt. 50%]composites |
| The variation of A.C. conductivity is as a function of the frequency for pure PPy, PPy/Sr3(AsO4)2 [wt. 20% & wt. 50%] composites is shown in Figure 6.a, 6.b and 6.c respectively. It is observed that, the conductivity is increases with the frequency, showing multiple phases of conductivity. It can also be seen that, the value of conductivity is increases up to 0.095 S cm-1 for Sr3(AsO4)2 [wt. 20%]. This may be due to the extended chain length of polypyrrole which facilitate the hopping of charge carriers when the content of Sr3(AsO4)2 is increased up to wt. 20%. The increase in conductivity for wt. 20% is due to the variation in distribution of Sr3(AsO4)2 particles which may be supporting for more number of charge carriers to hop between favorable localized sites causing increase in conductivity. And the conductivity of composites which was decreases for composites thereafter may be attributed due to the trapping of charge carriers. |
 |
| Variation of A.C. conductivity is as a function of the wt. % of Sr3(AsO4)2 in the pure PPy at different frequencies is shown in Figure 6.b. The value of the conductivity was increase up to 20 wt. % of Sr3(AsO4)2 in PPy. This weight percent is the percolation threshold for these composites. The composites obey percolation theory. This can be attributed due to the distribution of Sr3(AsO4)2 particles in PPy due to hopping of the charge carriers [9-10, 24-26]. |
IV. CONCLUSION |
| The PPy/Sr3(AsO4)2 composites were synthesized to tailor the transport properties. Detailed characterizations of the composites were carried out using SEM, FTIR, XRD and TG/DTA techniques. The results of A.C. conductivity of PPy/Sr3(AsO4)2 composites show a strong dependence on the weight percent of Sr3(AsO4)2 in polypyrrole. PPy/Sr3(AsO4)2 composites may find applications in the field of sensors. |
ACKNOWLEDGEMENT |
| The authors would like to acknowledge The Principal, BMSCE, BMSET, Bangalore-560019 for their cooperation and help. FTIR, TGA, SEM and XRD analysis of the samples were carried out at Sophisticated Analytical Instruments Facility (SAIF) of Cochin University of Science And Technology, Cochin, India. The author1 thank to wife and children for cooperation. |
References |
|