ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
|
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
An anionic diol, potassium-S-1,2-dihydroxyprppylsulphonate, (SDOL) has been synthesised by using phase transfer catalyst, benzyltriethylammonium chloride and subsequently used for the preparation of polyester ionomer. A series of polyester were successfully synthesised by using different molar ratio of the ionic diol and propane diol. The monomer (ionic diol) and polyester were characterised by FTIR, 1H NMR, TGA and DTG. The inherent viscosities of polyesters were in the range 0.38-1.21 dL/g.
Keywords |
| Polyester ionomer, benzyltriethylammonium chloride, ionic diol, potassium-S-1,2- dihydroxypropylsulphonate |
I.INTRODUCTION |
| Polyesters are a highly versatile class of polymers synthesized generally from a diacid and a diol. During the last 30 years, scientists have been fascinated by a class of polymers known as ionomers.Ionomers are normally defined as ion-containing polymers with a maximum content of ionic groups of about 15mol% or less along the backbone chains or as pendent groups. The modification and control of polymer properties through the use of ionic functional groups have received a considerable amount of attention in recent years. The common thread in most of the research efforts to date was the knowledge that the presence of even a small amount of covalently bonded ionic moieties in organic polymers is known to exert a profound effect on their physical and rheological properties. In fact ionomers have been shown to exhibit considerably higher moduli, and higher glass transition temperatures compared to their non-ionic analogues. Our research effort is directed towards the development of new polymer structures by introducing ionic groups (SDOL) into the polyester backbone. The first fibre-forming polyesters were prepared by Carothers and Hill in the late 1920s using the melt condensation of dicarboxylic acids and aliphatic diols. 1 |
| Ionomers are materials of great technological interest due to the fact that the ionic groups can interact with each other disturbing the supramolecular structure and modifying their physical and chemical properties.2-4 The most widely accepted model for ionic aggregation in ionomers is the Eisenberg-Hird-Moore (EHM) model.5 This model is best on the results obtained for polystyrene-carboxylate ionomers. Several examples of random and telechelic copolyester ionomers derived from poly(ethylene terephthalate) (PET) 6-7 or poly(butylenes terephthalate) 8-9 have been reported, where the main changes that were observed by the insertion of the ionic groups. With the aim at improving the thermal properties, in this article focus on the synthesis and spectroscopic characterisation of polyester ionomer. |
II.EXPERIMENTAL |
| A. Materials |
| Potassium sulphite, 3-chloropropane-1,2-diol, Diethyl ether, absolute alcohol were purchased from Sigma-Aldrich and were used as received. Benzyltriethylammonium chloride were used as received. Terephthaloyl chloride (TPC) and Isophthaloyl chloride (IPC) were purified by distillation under reduced pressure prior to use. Dimethyl sulphoxide, DMSO was dried over calcium hydride powder for 1 week and distilled prior to use. |
| Synthesis of Potassium S-1,2-dihydroxypropyl sulphonate:(SDOL)10,11: |
| Powdered potassium sulphite (4.748g) was dissolved in 10 mL water to which 3-chloropropane-1,2-diol (2.212g) added, followed by a further 10 mL of water. The solution was refluxed gently for 2h, added benzyltriethylammonium chloride (10mol %) and cooled. Unreacted 3-chloropropane-1,2-diol (CPD) were extracted with diethyl ether (2×2mL). The water was then removed by rotary evaporation under vacuum. After cooling, the product was extracted from the inorganic salt with hot DMSO (3×10mL). The DMSO extracts were centrifuged until clear and reduced in volume by rotary evaporation at 1000C to get viscous colourless oil. The oil was dissolved in 2mL of water. Absolute alcohol was added to the solution to form an emulsion from which product crystallized after standing for 2 hour at room temperature and then dried in vacuum desiccators. |
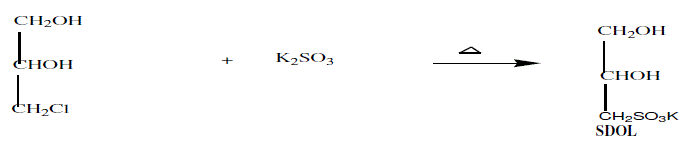 |
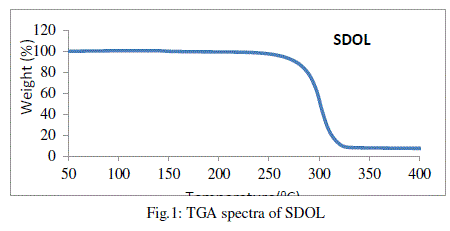
| SDOL was characterized by FTIR, 1H NMR and TGA spectral studies and the data were found to be in agreement with reported results. TGA was used to monitor the thermal stability of SDOL and the degradation temperature was found to be 2800C, which is the limit of temperature that could be used for synthesis of polyester ionomers with SDOL. |
 |
| B. Preparation of polyesters: |
| Polymerisation was carried out in a 250 mL two necked reaction flask equipped with a mechanical stirrer. In a typical polymerisation reaction, SDOL (0.776g,4 mmol), propane diol (0.30436g,4 mmol) and Triethyl amine (2×8 mmol, 1.61904g) was dissolved in 15 mL of water. Stirred the reaction mixture by using Heidolph overhead stirrer at 20,000 rpm for 45 minutes. After 45 min, solution of IPC (1.62416g, 8 mmol) in 20 ml of dichloromethane was added to the reaction mixture and the mixture was stirred vigorously for another 1 hour. The reaction mixture was then poured in hot water and methanol and the precipitated polymer was filtered and washed several times with water. The product was dried under vacuum. |
| C.Analytical Techniques |
| Inherent viscosity (ηinh) of polyester were measured using Ubbelohde viscometer in chloroform at a concentration of 0.5 g/ dL at (30±0.1)0C. Infrared spectra were recorded as KBr pellets on a PerkinElmer RXI FTIR spectrophotometer. 1H and 13C NMR spectra of the monomer and polyesters were obtained using 300 MHz spectrometer. The initial decomposition temperature (IDT) was determined from TGA curves of polyesters recorded on Mettler Toledo TGA/DSC1 STARe thermal analysis at a heating rate of 200C/min under nitrogen. |
III. RESULTS AND DISCUSSION |
| A series of polyester ionomer was synthesised by solution polycondensation from combinations of IPC, TPC, PD and SDOL in dichloromethane. FTIR spectra confirmed the formation of polyester. FTIR spectra of the polyesters exhibited absorption band for ester carbonyl in the range 1730-1740 cm-1. The effect of polymer structure on thermal properties of polyesters was studied by TGA. The monomer (ionic diol) synthesised was characterised by FTIR, 1H NMR, TGA. The inherent viscosity of the polyester were in the range 0.38-1.21dL/g. |
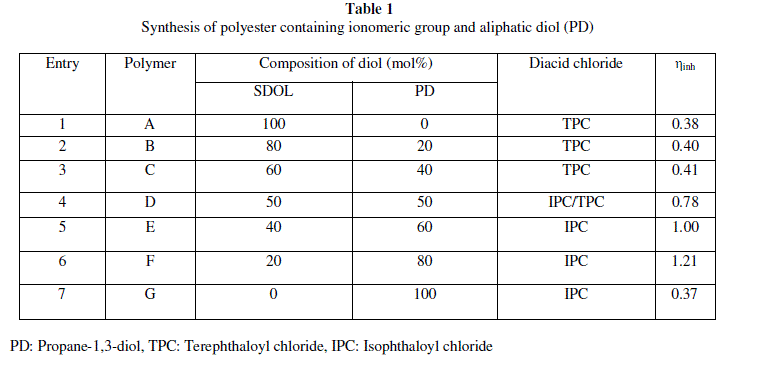 |
| PD: Propane-1,3-diol, TPC: Terephthaloyl chloride, IPC: Isophthaloyl chloride |
| A.TGA Spectra: |
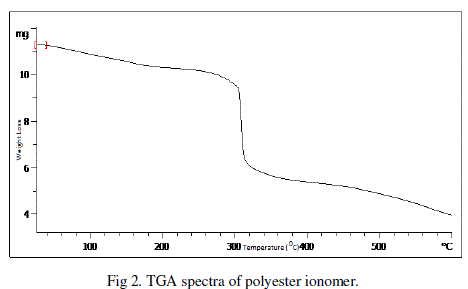
| The effects provoked by the incorporation of ionomeric units on the thermal properties were investigated by TGA. TGA measurements were carried out under nitrogen atmosphere . The initial decomposition temperature (IDT) of polyester ionomer was found to be 3000C. |
 |
| B. FTIR Spectra: |
| FTIR spectra of polyester confirmed the formation of polyesters where ionic groups at various mol % were used. The polyester exhibited absorption band for ester carbonyl in the range 1735 cm-1 which is in agreement with the reported results. |
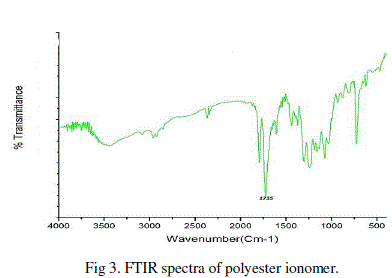 |
IV.CONCLUSIONS |
| The monomer was successfully synthesised by using phase transter catalyst, benzyltriethylammoniumchloride. The polyester ionomer were synthesised by optimising the reaction conditions and varying the different molar ratio of ionic diol and aliphatic diol (propane diol). Polyester ionomer were characterised by the spectroscopic technique like FTIR, TGA. Inherent viscosities of polyester ionomer were also measured. |
References |
|