ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
R.SharmilaDevi1,2, Dr.R.Venckatesh3, Dr.RajeshwariSivaraj4
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Nanosized Titanium dioxide (TiO2) powder was synthesized via sol-gel method using titanium tetraisopropoxide (TTIP) as the precursor. The as prepared nano powder was used for further characterization. The phase transformation was investigated by an X-ray diffractometer (XRD). The anatase structure of titanium dioxide was obtained after calcination. The microstructure was characterized by a Scanning Electron Microscope (SEM).
Keywords |
| Titanium dioxide(TiO2),Sol-gel method, X-ray Diffraction, Scanning Electron Microscope,FTIR. |
I. INTRODUCTION |
| Titanium dioxide (TiO2) is a very useful semiconducting transition metal oxide material and exhibits unique characteristics such as lowcost,easy handling, non-toxicity and resistance to photochemical and chemical erosion.These advantages make TiO2 a material in solarcells,chemicalsensors,for hydrogen gas evolution,aspigments,self-cleaning surfaces and environmental purification applications Hoffmann et al. [1]. The oxide nanoparticles synthesized by several methods appear more and more useful, because these nanoparticles have goodelectrical,optical and magnetic properties that are different from their bulk counterparts Wang et al. [2]. |
| Titanium dioxide exists in both crystalline and amorphous forms and mainly exists in three crystalline polymorphos,namely,anatase,rutile and brookite.Anatase and rutile have a tetragonal structure,whereasbrookite has an orthorhombic structureMahshid et al.[3].The immobilization of TiO2 nanoparticles on an appropriate support has been widely accepted since it could help to eliminate the costly phase separation processes and to promote the practicality of such catalysts as an industrial process. Recently,photocatalytic activity of immobilized TiO2 particles on macroporous ceramic alumina foams has been reportedPlesch et al. [4].It was found that reticulated macroporous ceramic foam with an open three-dimensional structure and low flow resistance improving the light penetration and fluid flow is highly promising support for photocatalytic applications and water purification systems. |
| TiO2 thin films have found application in dye-sensitized solar cells (DSSC) because of their interconnected pore networks and a large surface area,which allows sufficient dye adsorption and efficient light harvesting.Hence,the performance of such cells depends on the nature of porous structure and average particle size besides the phase. The conversion efficiency of ~11% or more is already attained in the laboratoryGratzeletal.[5].However, the commercial devices have yet to reach at that level. Cell stability is another issue requiring attention. One way to improve the efficiency and stability of DSSCs may be through the exploitation of electrodes with TiO2 nanoparticles of superior characteristicsHagfeldt et al.[6]. |
| Among the various photocatalytic materials that have been used, most attention has focused on titanium dioxide (TiO2) as a photocatalystin diverse areas ranging from water and air treatment to self-cleaning surfaces.Nanoparticles have been prepared by many methods such as Langmuir-Blodgett filmsYi et al. [7], vesiclesYoun et al.[8]and reverse microemulsionsFendler et al. [8].The chemical and physical properties exhibited by these materials depend,amongothers,on both the composition and the degree of homogeneity.Therefore, different synthesis strategies have been developed Toba et al. [9], Gao et al. [10].such as co-precipitation,flame hydrolysis, impregnation,chemicalvapourdeposition,etc.The sol-gel route has demonstrated a high potential for controlling the bulk and surface properties of the oxides (Ward et al. [11],Liu et al.[12], Schraml-Marth et al.[13]).Depending on the drying conditions the binary oxides could be obtained as aerogels, either by supercritical drying Pajonk et al.[14], Dusi et al. [15],or by silanization of the material previously to a conventional drying Sotelo et al. [16].Additionally, non-hydrolytic sol-gel routes have been also reported in the literatureHay et al.[17]. |
| Major research efforts have recently focused on the fabrication and characterization of nano-sized dielectric materials,because the current technology requires very small sized particles tominiaturise microwave devices and components. Particularly,TiO2 has been utilized in wide applications such as gas and temperature –sensing devicesKulwichi et al.[18], Micheli et al.[19] photocatalytic devicesLevy[20] and photoelectric devices Barbe et al.[21].Recently, TiO2 has attracted attention for use in fabricating capacitors in microelectronic devices due to its unusually high dielectric constant. Although the dielectric properties of bulk TiO2 having micron-sized grains have been well reported ,Noh et al.[22],those of TiO2 having nano-sized grains have received less attention because of the difficulties in fabrication. The modified dielectric properties were used in devices,such as capacitors,electronic memories and optical filters. The high dielectric permittivity and the low loss factors over a wide frequency range are always of great interestPrasad et al [23]. |
| The sol-gel method is an attractive method for the synthesis of titaniaZhou et al.[24].Since this method is carried out in solution,tailoring of certain desired structural characteristics such as compositional homogeneity,grain size, particle morphology and porosityis possible. A uniform distribution of the particles is important for optimal control of grain size and micro structure to maintain high reliability Siti Aida Ibrahim et al.[25 - 26]. |
| The focus of the present work is to synthesize high efficient nanosizedtitania particles having large surface area and to characterize the prepared sample using techniques like XRD,SEM,FTIR. |
II.RELATED WORK |
| Sol-gel is the most simple and sophisticated methodByun et al[27] among the various methods, in which various material parameters such as the powder morphology,surface area, average crystallite size and phase structure are controlled in determining photocatalytic activity of TiO2. TiO2 has band gap energy 3.2 eV. It is active in UV light,in which electrons are injected in to conduction band leaving behind holes in valence band. The sol-gel route is considered as very promising one for the synthesis of ultra-fine metallic oxide Li et al[28] and has been widely applied for preparing of TiO2 particlesHague et al[29]. For synthesis via sol-gel route several different percursors can be used. In the present study,we have prepared nano-sized TiO2from organometallic precursor- titanium tetraisopropoxide (TTIP) and characterized the prepared sample. |
III.EXPERIMENTAL DETAILS |
| A.Materials |
| Titanium Dioxide (TiO2) and glassware was purchased from Merck Chemical Reagent Co. Ltd. India. All glassware was washed with sterile distilled water and dried in hot air oven before use. |
| B. Preparation of TiO2 nanoparticles |
| In the synthesis of TiO2 particles,Titanium tetra isopropoxidewas used as a precursor and was mixed withHCl,ethanol and deionized water mixture,stirred for half an hour, in pH range of 1.5 . 10ml of deionized water was added to the above mixture and stirred for 2 hours at room temperature. Finally the solution was dried at temperature and the powder was heated at 120ºC for 1 hour. |
IV. RESULTS AND DISCUSSIONS |
| A.X-Ray Diffraction (XRD) Analysis |
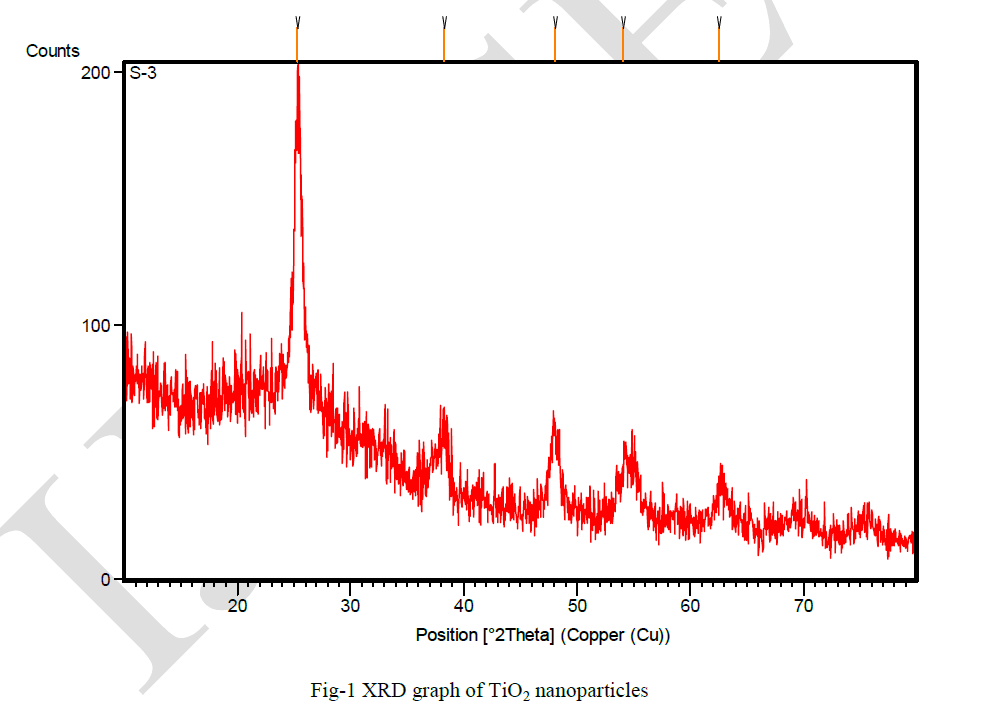
| The powdered sample was used by a Cu Kα - X Ray Diffractometer for confirming the presence of TiO2 and analyze the structure and shown in Fig. 1. The peaks appeared at 2θ value ranging The diffraction peak at 2θ with 25.3 o , 38.3 o , 48 o , 54 o , 62 o , corresponds to the crystal planes of (101), (004), (200), (105) and (204) respectively, indicating the formation of anatasephase of TiO2. The peaks of the graph are in good agreement with the literature report (Akarsuet al. [30].The location of the peaks was compared to literature values and the presence of Titanium dioxide particles was confirmed. The average size of the particles was calculated using Debye-Scherrer"s formula: |
 |
| B. Scanning Electron Microscope (SEM) Analysis |
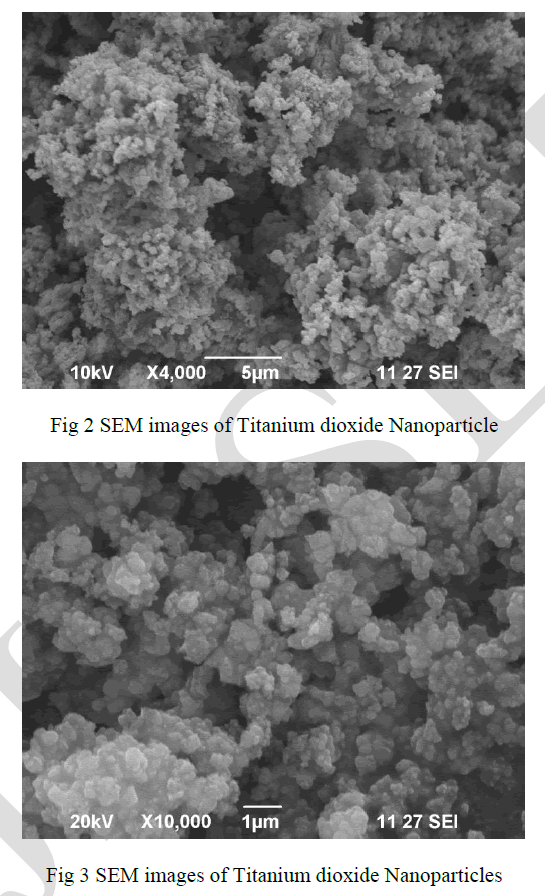
| The SEM analysis was used to determine the structure of the reaction products that were formed. Thin films of the sample were prepared on a carbon coated copper grid by just dropping a very small amount of the sample on the grid, extra solution was removed using a blotting paper and then the film on the SEM grid were allowed to dry by putting it under a mercury lamp for 5 min. SEM image hasroughly spherical spongy shape and agglomeration nanoparticles given in Fig. 2-3. |
 |
| C. FTIR Analysis |
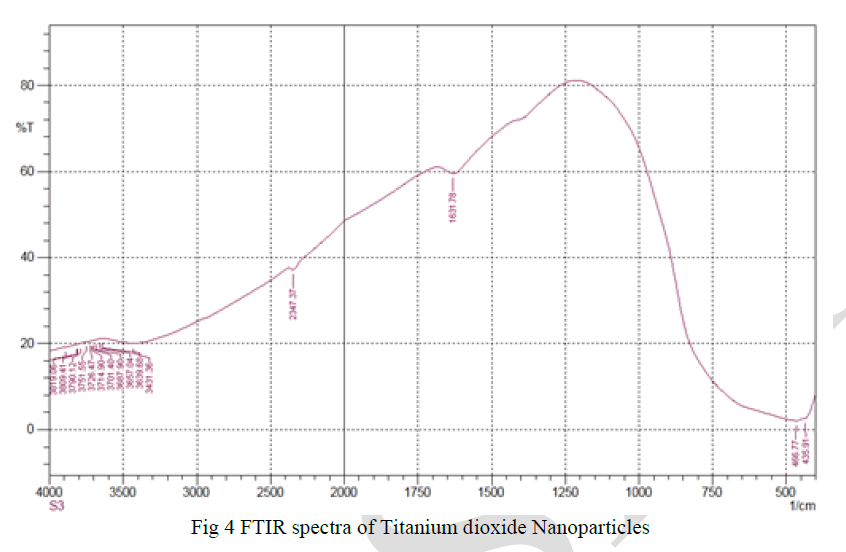
| FTIR analysis was used to determine the functional groups of titanium dioxide nanoparticles.In Fig.4. shows FTIR spectrum of titanium dioxide nanoparticles in whichthe peaks at 3400and 1631.78 cm-1, in the spectra are due to stretching and bending vibration of the –OH group. The peaks at 435.91cm-1, 466.77 cm-1to 700 cm-1 shows bending and stretching mode of Ti-O-Ti.There is no peak at 2900cm-1 which means all organic compounds removed from the samples after calcinations. |
 |
V. CONCLUSION |
| Titanium dioxide (TiO2) nanoparticles have been successfully synthesized using a wet chemical technique. The formation of the TiO2 nanoparticles was confirmed by powder X-ray diffraction (XRD). The size and morphology of the samples were characterized using scanning electron microscopy (SEM).The spherical shaped particles were confirmed through the SEM analysis. |
References |
|