e-ISSN: 2319-9849
e-ISSN: 2319-9849
Anant Babu Marahatta1,2* and Jagadeesh Bhattarai3
1Department of Chemistry, Amrit Science Campus, Tribhuvan University, Kathmandu, Nepal
2Department of Chemistry, GEMS Institute for Higher Education, GEMS School, Lalitpur, Nepal
3Central Department of Chemistry, Tribhuvan University, Kathmandu, Nepal
Received Date: 25/02/2019; Accepted Date: 02/05/2019; Published Date: 18/05/2019
Visit for more related articles at Research & Reviews: Journal of Chemistry
We investigated the corrosion behavior of sputter-deposited nanocrystalline W-Mo alloys with different atomic percentage in neutral medium at 25°C, open to air, by electrochemical measurements including weight loss method. We found comparatively low/high corrosion rate of W-Mo alloys having more/less percentage composition of tungsten. The open circuit potential and potentiostatic polarization measurements of such alloys estimated comparatively very short time required to achieve steady state, indicating rapid development of highly adherent and very stable thin oxide anodic passive film having high protective quality. The molybdenum enriched W-83Mo alloy demonstrates an active-passive transition with very narrow passive region and transpassive dissolution.
Corrosion, Nanocrystalline W-Mo alloys, Neutral Medium, Potentiostatic Polarization
Rusting or Corrosion is a destructive attack of a metal by chemical or electrochemical reaction with its environment [1]. According to IUPAC, corrosion is an irreversible interfacial reaction of the materials (metal and its alloys, ceramic and polymer) with its environment, which results in consumption of the material or the dissolution of material into the components of the environment [2].
According to electrochemical theory, corrosion process is a combination of anodic dissolution or oxidation and cathodic reduction. An acid-base reaction concept of corrosion [3] proposed to explain hydration or complexation of metal ions, however, is not widely discussed. As the metallic specimen is immersed in an electrolyte, the electrochemical reactions (two or more oxidation and reduction partial reactions) occur in such a way that the total rate of oxidation is equal to total rate of reduction as illustrated by Evans diagram in Figure 1.
A metallic dissolution (M→M n+ + ne-) usually occurs at anode. So, if a stable surface film is not formed, the dissolution rate measured in terms of anodic current density iA generally increases logarithmically with increasing potential E, as in Equation 1 and the rate of cathodic reaction involving hydrogen ions and / or oxygen dissolved in the solution (2H++2e−→H2; O2+2H2O+4e− 4OH− ; O2 + 4H+ + 4e−→2H2O) rises logarithmically with lowering E as in Equation 2. At the open circuit corrosion potential, both anodic and cathodic reactions occur simultaneously with the same rate [4], as in Equation 3.
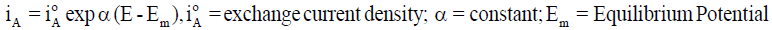 (1)
(1)
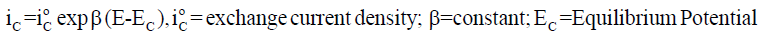 (2)
(2)
iA =iC (3)
The corrosion behavior of the materials often depends upon their structure, composition and metallurgy. In early 1960s, amorphous and supersaturated solid solution of metallic alloys prepared by rapid vapor quenching were found to possess technologically important physical, chemical, mechanical, magnetic, electronic and catalytic properties [5,6]. Sputtering, one of the main vapor quenching techniques, is mostly carried out for tailoring new corrosion-resistant metastable amorphous or nanocrystalline alloys with very fine grains (less than 20 nm) [7]. This technique is already been employed to prepare single phase amorphous or nanocrystalline Magnesium-transition metal [8] and Tungsten-transition metal alloys. The difficulties arise to prepare high concentration Tungsten based alloys by conventional way (due to very high melting point of tungsten: 3420°C) can also be solved by sputtering technique [9-13]. The sputter-deposited amorphous / nanocrystalline alloys are free from crystalline defects, such as grain boundaries and precipitates which act as nucleation sites during corrosion. They are of chemically homogenous single phase nature creating a uniform protective passive film on their surface, which separates the bulk from aggressive media, leads to extra corrosion resistance [14].
The Tungsten and Molybdenum are very effective alloying elements in enhancing the corrosion resistance of stainless steels in aggressive environments. Their small percentage (less than 1%) in alloy steels improves corrosion resistance as well as promotes fine grains [15]. Likewise, Molybdenum also promotes the formation of Chromium enriched protective film on the surface of stainless steels, adding strength on corrosion resistivity. These elements are not only used in alloy steels but also mostly used to manufacture the high corrosion resistance amorphous / nanocrystalline alloys by sputtering techniques. The sputter-deposited Tungsten-transition metal (Ti, Zr, Ta, Nb and Cr) [9-13]; Molybdenum-transition metal (Ti, Zr, Ta and Nb) [16,17] and Chromium-transition metal (Ti, Zr, Nb and Ta) [18,19] alloys are already found to demonstrate remarkably high corrosion resistance in aggressive media. In most of the cases, the corrosion resistance power of the alloys are found higher than those of alloy constituting elements in very aggressive media including concentrated HCl solution. The Tungsten-Niobium alloys, however, showed higher corrosion resistance even in less aggressive media (such as 10% NaCl and 10% NaOH solutions). The main cause of this high corrosion resistivity is explained elsewhere [9-20]: it is due to the rapid development of passive films on the surface of amorphous / nanocrystalline alloys.
The rapidly quenched single-phase amorphous/nanocrystalline sputter-deposited Tungsten-Molybdenum alloys (hereafter, W-Mo alloys) have been the subject of interest for many years as they demonstrate remarkably high corrosion resistance along with many novel properties. It has already been reported that the nanocrystalline sputter-deposited W-Mo alloys show high corrosion resistance than those of Tungsten and Molybdenum metals in 12M HCl solution [21-23]. Moreover, the co-author of this research article has highlighted the preliminary results about passivation behavior of W-Mo alloys in neutral medium (0.5M NaCl solution) at 25°C, open to air [24]. Thus, it is very much needful to report the detailed findings of in-depth research on corrosion behavior of W-Mo alloys in the neutral medium at 25°C, open to air. This work is mainly aimed at investigating corrosion/passivation behavior of sputter-deposited nanocrystalline W-Mo alloys in the neutral medium more thoroughly. Major objectives include the study of electrochemical behavior of W-Mo alloys with different percentage composition and ultimate estimation of their corrosion rates and passivation behavior in neutral medium at 25°C, open to air.
Sputter-deposited Nanocrystalline W-Mo Alloys
All the sputter-deposited W-Mo alloys used to conduct this research work (Table 1) were directly received from the co-author, Prof. Jagadeesh Bhattarai, Central Department of Chemistry, Tribhuvan University, Nepal. The sputtering apparatus and conditions and characterization techniques have been described elsewhere [21,23]. These alloys were characterized as: nanocrystalline structure having the apparent grain size of 20 nm or less, thickness of about 1.5-2.0 μm with wide composition range (atomic percentage, hereafter, at. %) as summarized in Table 1.
| Name of Alloy | Molybdenum content (at. %) | Tungsten content (at. %) | Apparent grain size (nm) |
|---|---|---|---|
| W metal | 0.0 | 100.0 | 17.0 |
| W-9 Mo | 9.0 | 91.0 | 17.0 |
| W-24 Mo | 24.2 | 75.8 | 19.8 |
| W-34 Mo | 34.2 | 65.8 | 16.0 |
| W-52 Mo | 51.8 | 48.2 | 15.0 |
| W-69 Mo | 69.3 | 30.7 | 18.5 |
| W-83 Mo | 82.8 | 17.2 | 16.5 |
| Mo metal | 100.0 | 0.0 | 20.0 |
Table 1. Chemical composition and apparent grain size of the sputter-deposited W-Mo alloys.
Corrosion Test
All the corrosion tests were conducted in 0.5 M NaCl solution prepared in distilled water at 25°C, open to air. Prior to corrosion test, the surface of each alloy specimen, that was cut into the pieces having area of 10-25 cm2, was mechanically polished with a silicon carbide paper up to grit number 1500 in cyclohexane, rinsed with acetone and dried by air blower. The immersion time of the alloys in 0.5 M NaCl solution was 98 hours. The corrosion rate was estimated by weight loss method, see Equation 4.
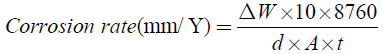 (4)
(4)
Where, ΔW = Difference in weight of specimens before and after immersion in 0.5 M NaCl solution (weight loss).
d=Density of alloy specimen (g/cm),
A=Area of alloy specimen (cm2),
t=Time of immersion (h).
The weight loss measurement of each alloy specimen was performed two times or more and an average corrosion rate was estimated.
Electrochemical Measurement
At first, the provided W-Mo alloys were cut into small pieces of 10-15 mm length and 6-10 mm in width. The surface of alloy specimens was treated as described in subsection 2.2. The alloy specimens were clipped by the crocodile pin welded with a stainless steel. About 60-100 mm2 area of each alloy specimen was immersed in an electrolyte and performed the electrochemical measurements. All the measurements were repeated two times or more.
Preparation of alloy specimens
At first, the provided W-Mo alloys were cut into small pieces of 10-15 mm length and 6-10 mm in width. The surface of alloy specimens was treated as described in subsection 2.2. The alloy specimens were clipped by the crocodile pin welded with a stainless steel. About 60-100 mm2 area of each alloy specimen was immersed in an electrolyte and performed the electrochemical measurements. All the measurements were repeated two times or more.
Electrolyte and electrodes
The electrolyte used for all the electrochemical measurements was 0.5 M NaCl solution, open to air and its temperature was controlled to 25 ± 1°C by using water bath. A saturated calomel electrode (hereafter, SCE) and a platinum mesh were used as the reference and counter electrode respectively. The alloy specimen was used as a working electrode. All the potentials given in this paper are relative to SCE.
Open circuit potential
The open circuit potential (hereafter, OCP) of each alloy specimen was recorded by using a potentiometer at 25°C, open to air. The potentiometer reading was noted immediately after immersing each alloy specimen in 0.5 M NaCl solution for 2 hours. All the OCP measurements were repeated two times or more.
Potentiostatic polarization
The potentiostatic polarization was carried out by using potentiostat (DT-2101, HI-TECK INSTRUMENTS ENGLAND). The potentiostat reading at each fixed potential was recorded immediately after immersing the sample into 0.5M NaCl solution as a working electrode for about an hour. Then the current density was calculated from immersion area of the sample at different potential values.
Corrosion Rate of W-Mo Alloys in Neutral Medium
The corrosion rate of each alloy specimen was estimated from the weight loss, see Equation 4, after immersion for about 98 hour in 0.5 M NaCl solution at 25°C, open to air. Figure 2 shows the change in corrosion rate of the examined W-Mo alloys with respect to at % of Mo. The corrosion rates of the sputter-deposited pure tungsten (at. % Mo=0) and molybdenum (at. % Mo=100) are about 1.8 × 10-2 mm/y and 6 × 10-2 mm/y respectively. These data are also plotted along with the corrosion rates of other W-Mo alloys, see Figure 2 for comparison. The decreasing trend of corrosion rate with at. % of W indicates that W greatly enhances the corrosion resisting power of Mo. The W-Mo alloys containing 9-52 at. % Mo have almost three times lower corrosion rate than that of the pure Mo (at. % Mo=100) and the corrosion rate of these alloys are slightly lower than that of the pure tungsten (at. % Mo=0) even after prolonged immersion in 0.5 M NaCl solution at 25°C. Interestingly, the corrosion resistance of the entire examined W-Mo alloys show lower corrosion rates than that of the molybdenum metal although its corrosion resistance will be enhanced by the addition of tungsten.
The nature of passive films formed on alloy surface plays a vital role for high corrosion resistance. Passivation of alloys occurs through their active dissolution at the initial periods of immersion in the solution. The initial high chemical reactivity of the alloys leads to the rapid accumulation of beneficial chemical species in the passive film developed on the surface. This accounts the high corrosion resistance of the alloys. Therefore, it is important to clarify time dependence (i.e., corrosion time) of W-Mo corrosion rate. In this work, we measured the corrosion rates of W-9Mo, W-24Mo, W-52Mo and W-83Mo alloys after immersion in 0.5 M NaCl solution at various time intervals as in Figure 3. The initial corrosion rate (up to 2 hour) of W-9Mo alloy is slightly higher than that of the W-24Mo and W-52Mo alloys. However, after immersion for about 10 hour or more, corrosion rate of W-9Mo alloy becomes lower than that of the other W-Mo alloys containing 24 to 83 at. % Mo. Moreover, the corrosion rates of all the examined W-Mo alloys became steady after immersion for about 22 to 100 hour. As a result, the average corrosion rate of the alloys increases with at. % Mo as shown in Figure 3.
Electrochemical Measurements of W-Mo alloys
The electrochemical measurements including OCP and potentiostatic polarization of the sputter-deposited nanocrystalline W-Mo alloys were carried out in 0.5 M NaCl solution at 25°C, open to air. The changes in OCPs as a function of immersion time is shown in Figure 4. The OCPs of all the examined alloys gradually decrease towards more negative direction with immersion time and attain steady state after 10-15 minutes. Moreover, the OCPs of all the examined alloys are in more noble direction than that of tungsten. These results revealed that more stable passive films are formed on the surface of the W-Mo alloys with increasing molybdenum content in the alloys.
The anodic passivity of the W-Mo alloys can be explained by potentiostatic polarization measurements. We measured potentiostatic polarization only for two alloys: W-9Mo and W-83Mo, because of the time and resource limitation. The change in anodic current density of W-9Mo alloy after potentiostatic polarization for 1 hour at different potentials: 600 mV, 800 mV and 1000 mV in 0.5 M NaCl solution at 25°C as a function of polarization time is shown in Figure 5. The anodic current density decreases with polarization time till about 2 minutes, then increases again up to 10 minutes and becomes almost steady, indicating the development of anodic passive films having high protective quality. Similarly, for W-83Mo alloy, the change in anodic current density after potentiostatic polarization for 1 hour at different potentials: 0 mV, 200 mV, 400 mV, 800 mV and 1000 mV in 0.5 M NaCl solution at 25°C as a function of polarization time is shown in Figure 6. It is obvious that the anodic current density increases with increasing potential, as it is seen in Figure 6 as well. Except at 0 mV (SCE), the current density does not show any significant variation with polarization time. At 0 mV (SCE), the current density decreases slightly with increasing polarization time up to 1 minute and becomes almost steady. This slight change in current density for 1 minute is mostly due to the formation of 0.5 M NaCl soluble Mo6+ ions. Then, further anodic dissolution is stopped due to the passive behavior of metal alloy. This passivity results from the formation of a highly adherent and very thin oxide film on the metal surface, which serves as a protective barrier to further corrosion. Such thin oxide passive film developed on the sputter-deposited W-Mo alloy surface might be the molybdenum oxyhydroxide, MoO(OH)3 [25], as it is insoluble in neutral solutions [26].
Figure 7 shows the potentiostatic polarization curve of the W-83Mo alloy after polarization for 1 hour in 0.5 M NaCl solution at 25°C, open to air. At low potentials, corrosion rates measured by anodic current density are very high. It causes the rapid development of passive film on the W-83Mo alloy surface. This passive film becomes more stable and corrosion rate falls to very low value as in Figure 7. The corresponding potential is called corrosion potential Ecorr It is also clearly seen that the W-83Mo alloy shows an active-passive transition in the potential range of -200 mV (SCE) and its passive region is in very narrow potential ranges. The passive potential Eρ which is defined as the potential of an electrode where a change from an active to a passive state occurs is also shown. The transpassivity is clearly seen above the potential -100 mV (SCE), as represented by transpassive potential Eρt mostly due to the formation of 0.5 M NaCl soluble Mo6+ ions.
We successfully investigated the corrosion behavior of sputter-deposited nanocrystalline W-Mo alloys in neutral solution at 25°C, open to air, by series of electrochemical measurements including weight loss method. We found an increment of corrosion rate of the W-Mo alloys with increasing molybdenum content (at. % Mo). In other words, the corrosion resistance power of W-Mo alloys increases with increasing tungsten content (at. % W). It is observed that the W-9Mo alloy, initially having higher corrosion rate than that of the W-24Mo and W-52Mo alloys, has lower corrosion rate later at prolong immersion. Attaining such rapid passivation is due to the accumulation of the beneficial chemical species in the passive film. The OCPs of all the examined sputter deposited W-Mo alloys are found to be in more noble direction than that of the tungsten. The potentiostatic polarization measurements revealed decrease in anodic current density with increasing polarization time at first and then increases for few minutes and becomes almost steady, indicating the development of anodic passive films, molybdenum oxyhydroxide, MoO(OH)3, having high protective quality. It could be very much appreciable and informative if we could collect the potentiostatic polarization data for all the six different W-Mo alloy specimens. Due to the time and resource limitations, this time, we carried the potentiostatic polarization measurements only for W-9Mo and W-83Mo samples. We observed that the molybdenum-rich W-83Mo alloy shows active-passive transition and transpassive dissolution. We claim that whatever methodologies we have followed in this research work are equally valid to study the corrosion behavior of any types of sputter-deposited nanocrystalline alloys in acidic/alkaline and neutral media. We believe that our research findings make some positive impacts in the production side of corrosion resistive nanocrystalline alloys including high speed steel or tungsten steel used in cutting tools. The same type of research work and analysis in future would be the boon to material science as well as the alloy manufacturing industries.
As mentioned in the experimental section, all the sputter-deposited W-Mo alloy specimens used in this research work were provided by Prof. Jagadeesh Bhattarai. Prof. Bhattarai prepared and characterized these alloys while working at Department of Chemistry, Tohoku University, Japan. The electrochemical and weight loss measurements were carried out at the Central Department of Chemistry, Tribhuvan University, Nepal. Dr. Himendra Jha, Atotech Berlin, Germany and Prof. Raja Ram Pradhananga, Tribhuvan University, Nepal are acknowledged for the constructive comments on this research article.