E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
Dagmar Mudronova, Viera Karaffová*, Radomira Nemcova, Viera Revajova, Sona Gancarcikova, Jana Koscova, Tomas Csank
University of Veterinary Medicine and Pharmacy, Komenskeho, Kosice, Slovak Republic
Received date: 21/02/2020; Accepted date: 05/03/2020 Published date: 12/03/2020
Visit for more related articles at Research & Reviews: Journal of Microbiology and Biotechnology
Probiotic bacteria as well as PUFAs belong to the well-known immunomodulators. In this work the effect of the application of probiotic lactobacilli in the form of probiotic cheese and flaxseed – a source of omega-3 PUFAs on the cell-mediated immune response in pigs was studied. 88 pigs were divided into 4 groups, where animals received during the period starting 10 days before weaning and lasting up to 21 days post-weaning: in the control group, a control cheese with sunflower oil, in group L probiotic cheese with sunflower oil, in group FA control cheese with whole crushed flaxseed, and in group LFA probiotic cheese with whole crushed flaxseed. The pigs were monitored for phagocytic activity, metabolic activity of phagocytes, proliferation of lymphocytes, proportions of lymphocyte subpopulations in peripheral blood and jejunal Peyer patches (PP). The immunomodulating effect of the application of probiotics and/or flaxseed was observed mainly on the local gut level. While on the day of weaning there were found to be no significant differences in proportions of observed lymphocyte subpopulations (CD4+, CD8+, CD3+, CD21+) isolated from jejunal PP, on the 7th day after weaning significantly higher percentages of T-lymphocytes were noted in L and FA groups in comparison to the LFA group. In contrast, the proportion of CD21+ was significantly higher in the LFA group as compared to all other groups. 21 days after weaning the proportion of PP lymphocytes was similar in all groups. The expected suppression of lymphocyte numbers and proliferation by flaxseed was not confirmed. Only phagocytic activity was inhibited after the addition of flaxseed, but the addition of lactobacilli eliminated this effect.
Cellular immunity, Flaxseed, lactobacilli, Probiotic cheese, Weaned pig
Post-weaning syndrome (PWS) is a serious health, breeding and economic problem for pig farmers. At weaning, exposure to a solid diet combined with other stresses, can lead to dramatic changes in the gastrointestinal (GI) microbiota, morphology and biochemistry, leaving piglets potentially susceptible to proliferation of pathogens and thus to the well-described PWS [1]. After the ban on the use of growth-promoting antibiotics in the EU in 2006, PWS cannot be controlled by those means, and consequently other safer alternatives to feed antimicrobials have been sought. Ways must also be found to improve the healthiness and safety of animal products reaching the consumer, and therefore safe natural substances (e.g. probiotics, polyunsaturated fatty acids- PUFAs, plant extracts, etc.) are at the centre of interest in this field.
The autochthonous GI microflora is considered to be one of the body’s natural defense mechanisms, and consists of a complex assembly of mostly non-pathogenic bacteria residing normally in the gastrointestinal tract (GIT). It plays an important role in the development of colonization resistance against pathogens. Stimulation of this autochthonous microbiota can be done by manipulating the composition of the diet, or adding of different natural components, especially probiotics [2-5]. The use of probiotics to enhance intestinal health has been proposed for many years. Probiotics possess well documented efficacy in the prevention and treatment of diarrhoeal diseases as well as modulation of immune response [6-9]. These beneficial effects have been explained by promotion of gut barrier functions.
Despite intensive research in this field, up to the present time probiotics are not such an efficient substitute for antibiotics. Therefore it is necessary to look for ways and means to increase their efficacy. One such way would seem to be combining the probiotic microorganisms with synergically acting components of natural origin (such as some oligosaccharides, PUFAs, phytocomponents or trace elements) which intensify the mode of action of the probiotic microorganisms or extend the range of beneficial effects of a probiotic preparation on the host [4].
Flaxseed (FS) is a rich source of PUFAs (above all omega-3), lignans and mucilaginous water-soluble polysaccharides, all of which have been found to confer numerous health benefits [10]. The stimulatory effect of omega-3 PUFAs on lactobacilli adhesion suggests that they could be used for enhancing the effectiveness of probiotics in inhibiting the digestive tract pathogens. These findings were demonstrated by Kankaanpää et al. [11] in in vitro experiments, by Ringo et al. [12] in fish and in our laboratory in pigs [4,13]. PUFAs are also known immunomodulators. The anti-inflammatory and anti-proliferative action of omega-3 PUFAs on the immune system cells is well documented in more studies, but some authors received also contradictory results [14-17]. The composition of PUFAs in the cells of the immune system indicates their potential effect on the biological receptors, signal transduction and lymphocyte proliferation. The consumption of diets high in fish-derived ω-3 PUFA, particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), has been demonstrated to reduce the inflammatory response [18], and alter immune parameters including antibody production and lymphocyte proportion and responsiveness [19- 21] in poultry and pigs.
The aim of this study was to investigate the effect of the administration of probiotic lactobacilli in the form of probiotic cheese and/or flaxseed as a source of omega-3 PUFAs and fiber on the cell-mediated immune response in weaned piglets. The effect of vaccination with circoviral vaccine was also studied.
Probiotic bacteria
The Lactobacillus probiotic strains were isolated at our Department of Microbiology and Immunology, University of Veterinary Medicine and Pharmacy in Košice. The Lactobacillus plantarum – Biocenol™ LP96 strain was selected from the gut contents of healthy suckling piglets. The Lactobacillus fermentum – Biocenol™ LF99 were isolated from the gastrointestinal tract of adult chicken.
Cheddar cheese was used as a vehicle for probiotic strains. The probiotic cheeses contained probiotic strains (each cheese one probiotic strain) at numbers 1 × 109 colony-forming units per gram (CFU/g) of cheese (referred to as probiotic cheeses). The probiotic bacteria were added to the cheese milk together with 2% starter culture Lactococcus lactis subsp. lactis (Lyofast MO 041 SACCO srl, Italy, freeze-dried) during typical Cheddar cheese production. The cheese that was used as a control, was a similar Cheddar cheese, but without the addition of probiotic strains (referred to as control cheese).
Animals, housing, diets and vaccination
The State Veterinary and Food Administration of the Slovak Republic approved the experimental protocol number 2108/07-221 and the animals were handled and sacrificed in a humane manner. The experiment was carried out on 72 clinically healthy piglets (crossbreds-Yorkshire x Pietrain). The piglets were housed in typical indoor pens and were divided into 4 groups of 18 animals each and the supplementation of their diets is shown in Table 1.
| Group | Supplementation-10 days before weaning up to 21st day after weaning |
|---|---|
| C (control) | Control cheese at a dose of 8 g/animal/day+sunflower oil (10% mixed in feed) |
| L | Probiotic cheeses A and B both at a dose of 4 g/animal/day+sunflower oil (10% mixed in feed) |
| FA | Control cheese at a dose of 8 g/animal/day+whole crushed flaxseed (10%mixed in feed) |
| LFA | Probiotic cheeses A and B both at a dose of 4 g/animal/day+whole crushed flaxseed (10% mixed in feed) |
| Note:Control cheese – without probiotic lactobacilli; Probiotic cheese A – Lactobacillus plantarum – BiocenolTM LP96; Probiotic cheese B – Lactobacillus fermentum – BiocenolTM LF99 | |
Table 1. Supplementation of the feed of piglets in the control and experimental groups.
Probiotic and control cheeses were supplied to piglets in the form of grated cheeses applied on the surface of feed. In the same period the animals had ad libitum access to water and feed mixture for early weaning of piglets OŠ-02 (CP 187.9 gm, ME 12.8 MJ, Fibre 38.3 gm, Lysine 11.6 gm, Methionine and Cysteine 6.4 gm, Threonine 7.6 gm, Tryptophan 2.3 gm, Choline 1352 mg, Vitamin A 11530 IU, Vitamin D3 1500 IU, Vitamin E 68.7 mg, Vitamin B2 7.1 mg, Vitamin B12 26.4 μg, Ca 7.5 g, P 6.2 g, Na 1.9 g, Cu 10 mg, Fe 163.4 mg, Zn 125.8 mg, Mn 72.7 mg; Spišské kÃâ¦Ãâ¢mne zmesi, Slovakia). A feed mixture was supplemented with whole crushed flaxseed-cultivar Flanders with high content of omega-3 PUFAs (Agrola KoÃâ¦Ã¾ušice, Czech Republic) for FA and LFA groups or with control oil (sunflower oil containing only omega-6 PUFAs) for control and L groups, both at a concentration of 10% (Table 2).
| Fatty acid | Flaxseed oil | Sunflower oil | Feed mixture |
|---|---|---|---|
| Lipids(dm basis) | 45.8 | ND | 2.2 |
| Palmitic, C16:0 | 5.1 | 6.3 | 17.4 |
| Stearic, C18:0 | 3.7 | 3.2 | 2.2 |
| Oleic, C18:1 | 18.4 | 22.6 | 24.7 |
| Linoleic, C18:2 | 16.1 | 67.9 | 51.9 |
| Linolenic, C18:3 | 56.8 | 0 | 3.8 |
| ND – not detected | |||
Table 2. Fatty acid compositions (in percentage) of flaxseed oil, sunflower oil and feed mixture OŠ-02.
The piglets were weaned at the age of 28 days. On the 29th day, six pigs from each group (which were subsequently sacrificed on day 21 after weaning) were vaccinated intramuscularly with 1 ml of porcine circovirus 2 (PCV-2) vaccine (Ingelvac® CIRCOFLEX™, Böehringer Ingelheim, Germany) as directed by the manufacturer.
Biological material
The pigs of all groups were sacrificed with T61 (Intervet International B.V. Boxmeer, The Netherlands, doses: 0.3 ml/kg body weight) intracardially on the day of weaning (28-days-old piglets) and on days 3, 7 and 21 post-weaning. The gastrointestinal tract was immediately removed from the sacrificed piglets.
Isolation and purification of lymphocyte subsets from the jejunal Peyer´s patches (PPs) for the FACS analysis have been performed by the modified method of Solano-Aguilar et al. [22]. Briefly, PPs were cut out from jejunum, washed in ice-cold PBS (137 mM NaCl, 2.7 mM KCl, 6.4 mM Na2HPO4, 1.2 mM KH2PO4, pH 7.3) and placed in ice-cold Hanks balanced salt solution – HBSS (137 mM NaCl, 5 mM KCl, 1.1 mM Na2HPO4.2H2O, 0.4 mM KH2PO4, 5 mM D-glucose, 4 mM NaHCO3, 10 mM HEPES, pH 7.1 – 7.3, filtered 0.22 μm). PPs were incubated in 30 ml HBSS-Dithiothreitol (2 mM dithiothreitol (Sigma) in HBSS) in a water bath at 37°C for 20 minute. Then samples were vortexed and media were removed. PPs were resuspended in HBSS-EDTA (1 mM EDTA (Lachema, Czech Republic) in HBSS) and incubated in a water bath at 37°C for 20 minutes. After incubation media were removed, PPs were immersed in a small amount of HBSS in a Petri dish and mucosa was scraped with the help of a plastic disposable cell scraper (Costar, USA). The suspension was filtered through a 70 μm nylon cell strainer (BD Falcon, USA) into 50 ml conical tube and HBSS was add to 30 ml. Tubes were centrifuged at 600 xg for 10 minutes. The pellet was resuspended with 8 ml of a 40% Percoll (Sigma) solution in HBSS (Percoll stock solution: 9 parts of Percoll+1 part of HBSS), then the suspension was divided into two 15 ml glass tubes and underlayed with an equal volume of 70% Percoll solution in HBSS. Tubes were centrifuged at 1000 x g for 30 minutes. Lymphocytes were collected from the 40-70% layer interface into a new tube. Cells were washed twice with HBSS by centrifugation at 600 × gm for 5 minutes, then counted after the staining with Türk solution in a Bürker chamber and adjusted at 1.106 cells per 50 μl.
The peripheral blood was collected from the retroorbital venous sinus into the tubes containing different anticoagulants (heparin for flow cytometric analyses, and natrium salt of EDTA for lymphoproliferative test). Leukocytes for the lymphoproliferation test were isolated from peripheral blood by the modified method of osmotic shock of erythrocytes [23].
Immunological analysis
Flow cytometric analysis of the lymphocyte subsets in blood or isolated from the jejunal mucosa were performed as follows: 50 μl of blood or isolated lymphocytes containing approx. 1.106 cells were incubated for 15 minutes in dark at laboratory temperature with primary monoclonal antibody (2 μl CD4a, 2 μl CD8a, 10 μl CD45, 1 μl CD3ε-FITC or 1 μl CD21-RPE). After incubation, 800 μl lysing solution (8.29 g NH4Cl, 1 g KHCO3, 0.393 g Na2EDTA to 1L H2O) were added to the tubes with non-conjugated MoAb (CD4, CD8 and CD45) and 1 ml BD FACS Lysing Solution (BD Biosciences, USA) were added to the tubes with conjugated MoAb (CD3ε-FITC and CD21-RPE). Then tubes were incubated for 20 minutes in the dark at laboratory temperature. Tubes were twice washed with 0.5 ml PBS (250g for 5 minutes). For CD4a, CD8a and CD45, 25 μl of secondary antibody diluted 1:50 in PBS was added and the tubes were incubated for 15 minutes in the dark at laboratory temperature. The tubes were again washed twice with 0.5 ml PBS (250g for 5 minutes) and 200 μl of 1% paraformaldehyde in PBS were added to the each tube. For analysis the following monoclonal antibodies were used: mouse antiporcine CD4a MCA 1749 (AbD Serotec, UK), mouse antiporcine CD8a MCA 1223 (AbD Serotec, UK), mouse antiporcine CD45 MCA 1222 (AbD Serotec, UK), mouse antiporcine CD3ε-FITC conjugate BB23- 8E6 (SouthernBiotech, USA), and mouse antiporcine CD21 R-phycoerythrin BB6-11C9.6 (SouthernBiotech, USA). As secondary antibody was used FITC conjugated polyclonal goat antimouse IgG (Sigma, USA). Flow cytometric analysis was performed on BD FACS CantoTM flow cytometer (Becton Dickinson Biosciences, USA) using BD FACS DivaTM software. Fluorescence measurements were carried out using the 488 nm blue laser with FITC filter (530/30 nm) or RPE filter (585/42 nm). Proportions of lymphocytes in PP are expressed in percentage, and those in blood as absolute numbers calculated from total number of CD45 positive leukocytes.
Phagocytic activity of monocytes and neutrophils was analyzed by the help of Phagotest® (ORPEGEN Pharma, Germany), metabolic burst activity of phagocytes by the Bursttest® (ORPEGEN Pharma, Germany).
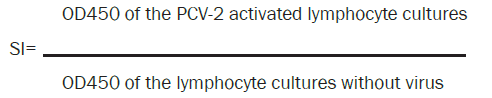
Stimulation Index (SI) of peripheral blood lymphocytes after stimulation by PCV-2 was measured by the lymphoproliferation test (LPT). A colorimetric immunoassay was used to quantify lymphocyte proliferation, based on the measurement of 5-bromo- 2'-deoxyuridine (BrdU) incorporation during DNA synthesis (Cell Proliferation ELISA Kit, BrdU-colorimetric, Roche Diagnostics, GmbH, Germany). Briefly, suspensions of leukocytes in 100 μl of 2 x 106 cells/well in RPMI 1640 with 10% of foetal calf serum (PAA Laboratories, Inc) were cultured 72 h with 50 μl virus PCV-2 in titer 104.3/ml (strain PCV-2 Stoon 1010; kindly provided by Prof. Gordon Allan, Agri-Food and Biosciences Institute, Belfast), and without virus in a 96-well microtiter test system at 37°C in a humid atmosphere at 5% CO2. There were five replicates of each treatment. Twenty four hours before the end of the cultivation, BrdU at the final concentration of 10 μM was added and the cells were reincubated for 24 hours. After removing the culture medium, denaturation of DNA and fixation of the cells on the bottom of wells, 100 μl of anti-BrdU-peroxidase labelled conjugate was added and allowed to react for 90 minutes at 25°C. The immune complexes were detected by the subsequent substrate reaction (100 μl L substrate solution) for 30 minutes at room temperature. The reaction was stopped by 25 μl 1 M H2SO4 and the optical density (OD) was measured in an ELISA-multiwell reader (BIO-RAD Laboratories, Inc., USA) at 450 nm (OD450).
The cell activation rate was expressed as a stimulation index (SI) calculated on the basis of the ratio of absorbance of the stimulated cells to the non-stimulated cells according to formula:
All analyses were performed using the statistical program GraphPad PRISM version 3.00. Tukey´s and Dunnett´stests (after analysis of variance, ANOVA) were used to identify the differences between groups.
Local gut immune response
While on the day of weaning there were found no significant differences in proportions of observed lymphocyte subpopulations (CD4+, CD8+, CD3+ and CD21+) isolated from jejunal PP, on the 7th day after weaning significantly higher percentages of T-lymphocytes (CD3+, CD4+, CD8+) were noted in L and FA groups in comparison with LFA group (Figures 1-3). In contrast, the proportion of CD21+ on 7th day was significantly higher in the LFA group as compared to all other groups (Figure 4). 21 days after weaning, percentage of T-lymphocytes was the lowest in L group, whereas percentage of CD21+ lymphocytes in PP was the highest in this group.
Total cellular immune response
Phagocytic activity was the lowest in the FA group throughout the experiment (Table 3). The highest phagocytic activity in the control group on 7th and 21th day after weaning was accompanied by a longer duration of diarrhoea. Similarly, higher values (p<0.05) of metabolic burst activity of phagocytes were found in the control on 7th and 21th day after weaning as compared to experimental groups (data not shown). SI of lymphocytes measured after stimulation with PCV2 in PCV2-vaccinated pigs was significantly higher in all experimental groups in comparison with control, but no significant differences were found among experimental groups (Figure 5).
| Sampling | control | L | FA | LFA | ANOVA |
|---|---|---|---|---|---|
| Weaning | 76.9 ± 2.39b*** | 82.9 ± 2.26b***c* | 61.0 ± 4.56 | 77.8 ± 3.27b*** | p<0.0001 |
| 7th day | 86.8 ± 2.13b** | 78.5 ± 7.54b* | 64.6 ± 9.26 | 74.7 ± 4.75 | p=0.003 |
| 21st day | 83.5 ± 4.85a*b* | 76.9 ± 3.14 | 69.1 ± 4.04 | 71.1 ± 6.91 | p=0.016 |
| a-significantly different from group LFA; b-significantly different from group FA; c-significantly different from control; *-p< 0.05; ** - p<0.01; *** -p<0.001 b - significantly different from group FA c - significantly different from control * - p < 0.05; ** - p < 0.01; *** - p < 0.001 b - significantly different from group FA c - significantly different from control * - p < 0.05; ** - p < 0.01; *** - p < 0.001 | |||||
Table 3. Effect of probiotic lactobacilli and flaxseed on phagocytic activity in peripheral blood (n=6).
In blood similar proportions of CD21+ and CD8+ lymphocytes were found as compared to lymphocytes isolated from the jejunal Peyer patches, but the differences between groups were not so pronounced (data not shown). Absolute numbers of CD21+ were significantly influenced only on the day of weaning, where lower numbers in the L group than in the control group (p<0.01) were found. The highest absolute numbers of CD8+ were noted in group FA on the day of weaning, when the numbers were significantly higher in comparison to L and LFA groups, and on the 7th day after weaning in comparison to L group.
Since on the day of weaning no significant differences were recorded in absolute numbers of CD3+ lymphocytes in blood, on the 7th day after weaning there were significantly higher numbers in FA and LFA groups in comparison with L (p<0.05 and p<0.001) and control group (p<0.05), and on the 21th day the highest absolute number (6.9 ± 2.2 × 106/ml) was in FA group, but also numbers in L and LFA groups were significantly higher than in control (p<0.05-0.001). Absolute numbers of CD4+ lymphocytes corresponded with proportions of CD3+ lymphocytes. CD4:CD8 ratio was in correlation with the highest numbers of CD4+ lymphocytes, what means significantly higher ration in the control group on the day of weaning (p<0.01-0.001) and on 21st day (p<0.001), and on 7th day in LFA group (p<0.05-0.001).
The weaning process in an intensified pig production system brings many sudden changes in the environmental and physical factors in a piglets’ life. These sudden changes, especially in diet combined with other stresses, can negatively disturb the immune function and the intestinal microbiota equilibrium of the pigs [24].
There is a strongly-held belief that dietary PUFAs significantly affect health, in part by modulating the immune system. The data from many animal studies have shown that fatty acids, concretely omega-3, can possess anti-inflammatory and anti- proliferative action, but some studies evidenced no or contradictory results [14,16,25]. In our experiment we have found significantly decreased phagocytic activity in group, where flaxseed was administered, but the percentage of lymphocytes isolated from the jejunal PP as well as absolute numbers of subpopulations of lymphocytes in blood were not decreased in this group. Existing evidence indicates that dietary fatty acids can modulate immune responses through one or more of three major molecular mechanisms: altered membrane composition and function, modified eicosanoid production, and changed cytokine biosynthesis [16]. Changes in membrane-dependent functions, such as phagocytosis is thougt to be a direct consequence of alterations in membrane composition and fluidity caused by addition of PUFA [26]. Also inhibition of some cytokines (IL-1, TNF-α and IFN-γ), which are known stimulators of PMNL, by n-3 PUFAs can cause decrease of phagocytic activity [27]. Decreased phagocytic activity after application of flaxseed in our experiment is in agreement with the results of Thies et al. [28], who administered 5% of fish oil rich in n-3 PUFAs to the diet of weaned pigs. However, in some studies the inhibitive influence of n-3 PUFAs on the phagocytic activity in pigs wasn’t noted [29-31]. The inhibitive effect of n-3 PUFAs on the proliferation and reactivity of lymphocytes described in more studies [28,32-34] was not confirmed in our experiment, similarly to the results of Thies et al. [28]. On the contrary, we noted a certain degree of activation of some populations of lymphocytes, especially CD3+ and CD8+ subsets.
Increased proportions of these subsets were observed in the jejunal PP as well as in the peripheral blood in the flaxseed group and were accompanied by the lowest percentage of CD21+ in PP, but not in blood on the 7th day after weaning. In contrast to cited studies, in which authors used only oils rich in n-3 PUFAs, we used whole crushed flaxseed, which also contains other biologically active compounds, especially the lignan precursor secoisolariciresinol diglycoside (SDG), and dietary fiber. Babu et al. [35] in their work compared the effects of whole and defatted flaxseed on lymphocyte proliferation. Defatted flaxseed or low concentration of flaxseed had no effects on lymphocyte proliferation, while a high flaxseed diet (40% of diet) was inhibitory. Since many studies have shown that supplementation of different antioxidants significantly increased lymphocyte proliferation [36-38]. Rhee and Brunt [39] speculated that also SDG isolated from flaxseed hulls and having an antioxidant activity could possess the same effect. They performed testing of SDG in in vitro conditions, in contrast to several previous studies, and no significant effect of SDG on lymphocyte proliferation was observed. In the current study, proliferation of lymphocytes tested ex vivo was slightly higher in the flaxseed group than in other groups, but this difference was not significant. More importantly from the clinical point of view, are the distribution and numbers of lymphocytes in different compartments of the organism, and these parameters were significantly influenced not only after supplementation of flaxseed, but also by the addition of lactobacilli or their combination. Flaxseed dietary fiber, in particular its soluble fraction containing polysaccharides, as was described above, can positively influence the composition of intestinal microbiota in favour of beneficial lactobacilli or bifidobacteria. Most probiotic bacteria belong to these two species and one of the modes of action of probiotics is also their immunomodulatory activity. Since most antigens enter the body through the mucosa, the mucosal immune system plays a crucial role in the defense response to pathogens. The intestinal microbiota is the largest source of microbial stimulation that can induce both harmful and beneficial influence on the health, and thus it participates in the development of the postnatal immune system as well as oral tolerance and immunity [40]. Beneficial bacteria joins in immune exclusion and protects the host from the adhesion of pathogens, they stimulate the production of specific antibodies, influence the distribution and the numbers of lymphoid cells in lymphatic tissues associated with the gut, ensure the balance in the composition of the gut microflora, and through their activity are able to maintain the integrity of the gut mucous membrane [41].
In group, where lactobacilli were administered, the higher phagocytic activity as compared to the flaxseed group was noted. In the group where pigs received a combination of probiotic lactobacilli and flaxseed, phagocytic activity in peripheral blood was higher than in the flaxseed group, but lower than in L group. These results are in accordance with many other studies [42-44]. The results from different studies on animals show that the increased function of phagocytic cells depends on the species or strain of bacteria. The strains able to survive in the gastrointestinal tract, able to adhere to the intestine mucous membrane, and able to persist at the critical limit are more effective in stimulating phagocytic cells [45,46]. As showed in our microbiological results, our strains of lactobacilli possess good colonization properties what can positively influence not only activation of phagocytosis but also their total immunomodulatory action [13,47].
From the health point of view, the crucial time is the first week after weaning. In this period we can observe the most important changes in weanling physiology and also in the immune response of the pig. It has an influence on mortality, which is the highest during this time. The most significant changes in proportions of lymphocytes in the jejunal PP as well as in blood were observed just on the 7th day after weaning. The addition of lactobacilli increased proportions of CD3+ and CD4+ subsets on the local gut level, but not in blood on day 7 after weaning. Only on the 21st day after weaning did we record a significant increase of CD3+ lymphocytes in blood. Similarly to our study, where we administered L. plantarum and L. brevis, also Perdigon et al. [48] demonstrated that L. plantarum was able to interact with Peyer’s patch cells and showed an increase in CD4+ cells. Interesting results were received in combined LFA group, where on the 7th day we found the highest proportions of CD21+ lymphocytes in the jejunal PP as well as in blood. In PP, this increase was accompanied by the lowest percentage of T-lymphocytes (CD3+, CD4+ and CD8+), but in blood numbers of T cells were not decreased, contrary CD3+ and CD4+ subsets were the highest in this group. Differences in proportions of the same subsets in the gut and blood can be explained by the redistribution of immune cells between gut associated lymphoid tissues and blood, based on the needs of the organism, because all compounds of the immune system, present locally, are in relationship to the whole of the organism [40]. Activation or suppression of different lymphocyte subsets by the action of probiotic bacteria is also species- or strain-dependent and are in detail reviewed in works of Delcenserie et al. [40] and Herich and Levkut [41].
In conclusion, the current study has shown that inhibited activity of phagocytes caused by flaxseed was eliminated by the addition of lactobacilli, moderate to significant activation of the local gut as well as total cellular immune response, and improved effect of circoviral vaccine was observed after addition of probiotic lactobacilli and/or flaxseed into the diet of weaned pigs.
This study was supported by the project of the Research and Development Operational Programme funded by the ERDF, by the EU Structural Fund ITMS 26220220185 (MediPark).