ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
K. Bansiwal1, N. Rai2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Malathion is an organophosphate insecticide and used as a test chemical to assess its toxicity for male reproductive organ of Eisenia foetida. Worms exposed to high dose (12000mg/Kg soil of 5 percent Malathion) for 96 hours showed significant changes in ultra structure of male organ of E. foetida. These changes include vacouliged cytophore and spermatids, deshaped mid piece of spermatid. It is suggested that Malathion at 12000mg/Kg soil for 96 hours has caused deformities at ultra structure level in male reproductive organ of E. foetida that will ultimately lower the reproductive rate of E. Foetida. Thus it can be said that Malathion is not only a neurotoxin agent but also a cytotoxic agent that also affects the other organs apart from nervous system.
Keywords |
| Malathion, testes spermatid, ultra structure |
INTRODUCTION |
| Organophosphates are insecticides, exerting their toxicity by binding to acetyl cholinesterase, inhibiting the action of this enzyme (which degrade the neurotransmitter acetylcholine), and eventually killing the organisms ([1], [2], [3]). Organophosphates are frequently used as pesticides due to their low half life. Malathion has been used for present investigation since it is commonly used in agriculture for killing sucking and chewing insect etc. Malathion is considered one of most innocuous organophosphates for humans, which led to an indiscriminate use of this pesticide everywhere. Among soil organisms, earthworms are particularly selected for the assessments because of these organisms play a vital role in vermi-composting and nutrient cycling and they also constitute a big part of the invertebrate biomass in the soil. Earthworms are the most suitable organism for use as key bioindicators organisms for testing the toxicity of chemicals in soil ([4], [5], and [6]) and also adopted as a standard organism for ecotoxicological testing ([7], [8], [9]). Furthermore, among the various earthworm species, Eisenia foetida is especially appropriate for the toxicity tests because it can be easily reared on a variety of organic wastes with short lifecycle. Its susceptibility to chemicals resembles that of true soil organisms. Sensitivity tests reveal that E. foetida is comparatively less sensitive than other earthworm species ([10], [11], and [12]). It is a suitable test model species for toxicological experiments [13]. Malathion-induced morphologic alterations have been found in the mouse testes [14]. According to reference [15] in laboratory rats the organophosphate causes a decrease of testicular mass with degenerative injuries and partial failing of the spermatogenesis and an increase in abnormalities of spermatozoa. In birds, Malathion induces germ cell degeneration possibly by altering cholinergic functions [16]. Worms makes direct contact with the ground and absorbs pesticides both from skin and digestive system, thus the pollutants are absorbed 5 to 10 times. The toxic effects on earthworm gametes should correspond directly to their population and indirectly to other wildlife [17]. The present work evaluates the changes produced after treatment of Malathion in the male reproductive organ of Eisenia foetida at intra cellular level discussed in the form of electron micrographs. |
II. MATERIALS & METHODS |
| 1. Experimental model |
| 2. Pesticide |
| 3. Experimental set-up |
| 4. Treatment of worms |
| 5. Electron microscopy of clitellum |
| 1. Experimental Model: Earthworms, E. foetida was chosen to evaluate toxicity in present study because it was easy to handle and its easy availability. Earthworms were procured from the vermicomposting unit of Rajasthan College of Agriculture, Udaipur. They were maintained in the laboratory condition, after 15 days acclimatization they were used for further conducted experiment. The worms used in the experiment were of approximately same body weight and body length. 2. Chemical: The pesticide used in the experiment was Malathion (5%). It was purchased from the local market's shop. The LD50 value of this pesticide for the E. foetida was evaluated by author first that is 16000mg/kg of soil and the result of this LD50 supported by the reference [18]. 3. Experimental set-up: Our method of the experiment was based on the method as in reference [19]. We used plastic tub for our experiment. Dried soil (from nearby farmland) was crusted and filtered through a fine mesh sieve. One kg of fine soil was than poured in each plastic tub and water was added to moistened the soil and 250 mg dried powdered (3 week old) cow dung was also added to each plastic tubs. Cow dung was added to avoid starvation. 20 mature earthworms (some age group) were added to each plastic tub. Each plastic tub was covered with muslin cloth. Thus one control set and one experimental set were prepared 3 replicates were used for each set. 4. Treatment of the worm with Malathion: Higher sub lethal lose of Malathion (12000mg/kg soil) was selected to study the toxicity of Malathion for E. foetida. Thus in experimental set higher sub lethal dose of Malathion was added and mixed throughoughtly in the plastic tub. Regular water supply was done to maintained 60% to 70% moisture level. After 96 hours of the treatment the worms were taken from the plastic tub and dissected out for desired organ of the body. 5. Electron microscopy of Testes: Worms were dissected from 9 to 11 segment from both the group (control and experimental) and immersed in Karnovsky fixer (1% glutaraldehyde and 4% paraformaldehye in 0.2 M (cacodylate buffer at pH 7.4). Then washed in 0.2M Cacodylate buffer (pH 7.4) and post fixed in 1% Osmium tetroxide, similarly buffered, for 1h following dehydration in an acetone series, the tissue were embedded in resin. Ultra thin sections were stained with uranyl acetate followed by lead citrate, examined and photographed with a Morgagni 268D, Transmission Electron Microscope. |
III. RESULTS AND DISCUSSION |
| In Eisenia foetida, spermatogenesis begins in testes where spermatogonia divide mitotically and with an incomplete cytokinesis originating a common cytoplasmatic mass. The cytophore which connects polarizes and synchronizes the surrounding cells through cytoplasmic bridges. From there, the whole spermatogonia move to the seminal vesicles where spermatogenesis process is completed. In the lumen of the vesiculae seminalis, cytophore, coelomcytes, and different stages of developing spermatocytes, and different stages of developing spermatozoids are floating. A central common nutrient anucleate mass of cytoplasm is known as cytophore. It consists of numerous mitochondria, clusters of ribosomes, and elaborate stack of parallel smooth endoplasmic reticulum, rough endoplasmic reticulum cisternae, membrane-bound dense bodies and degenerated dictyosomes (Fig.1 & 2). |
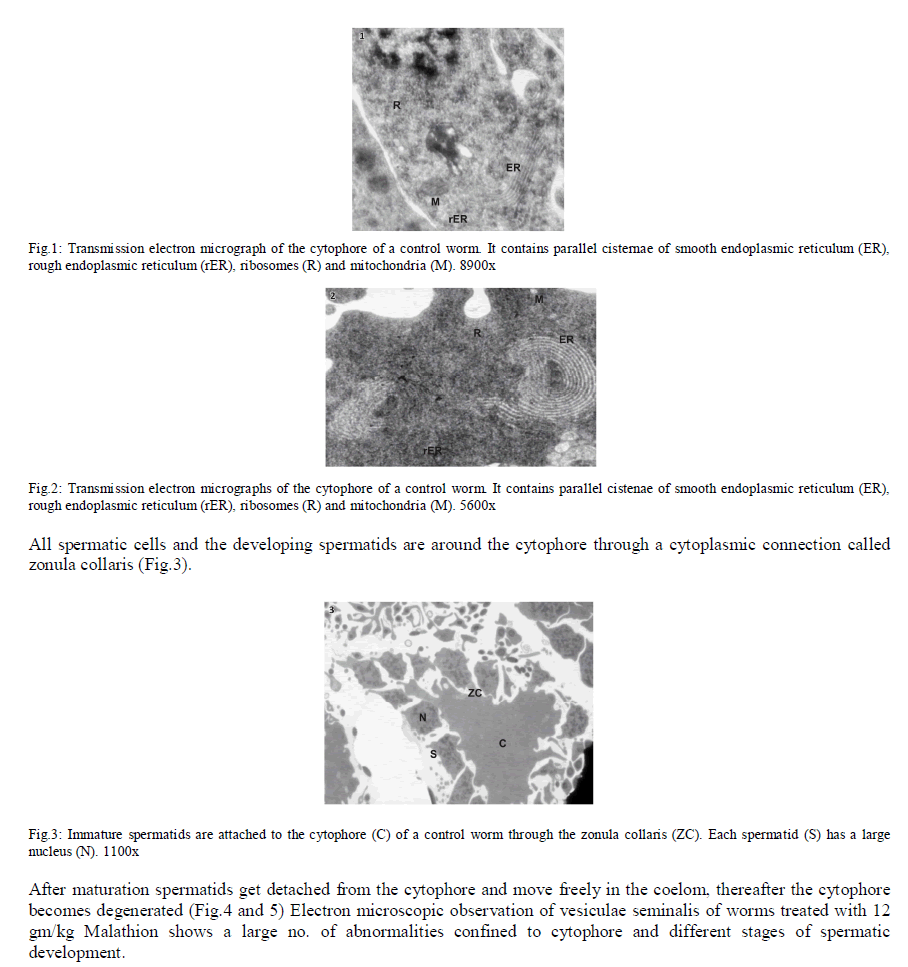 |
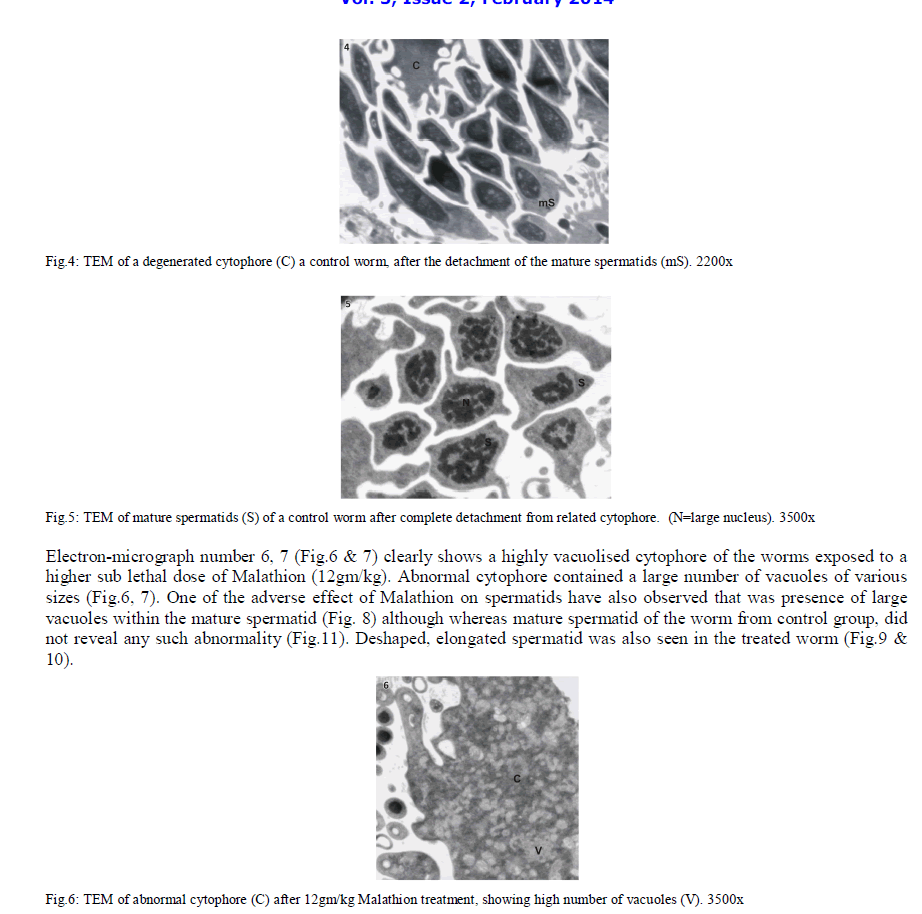 |
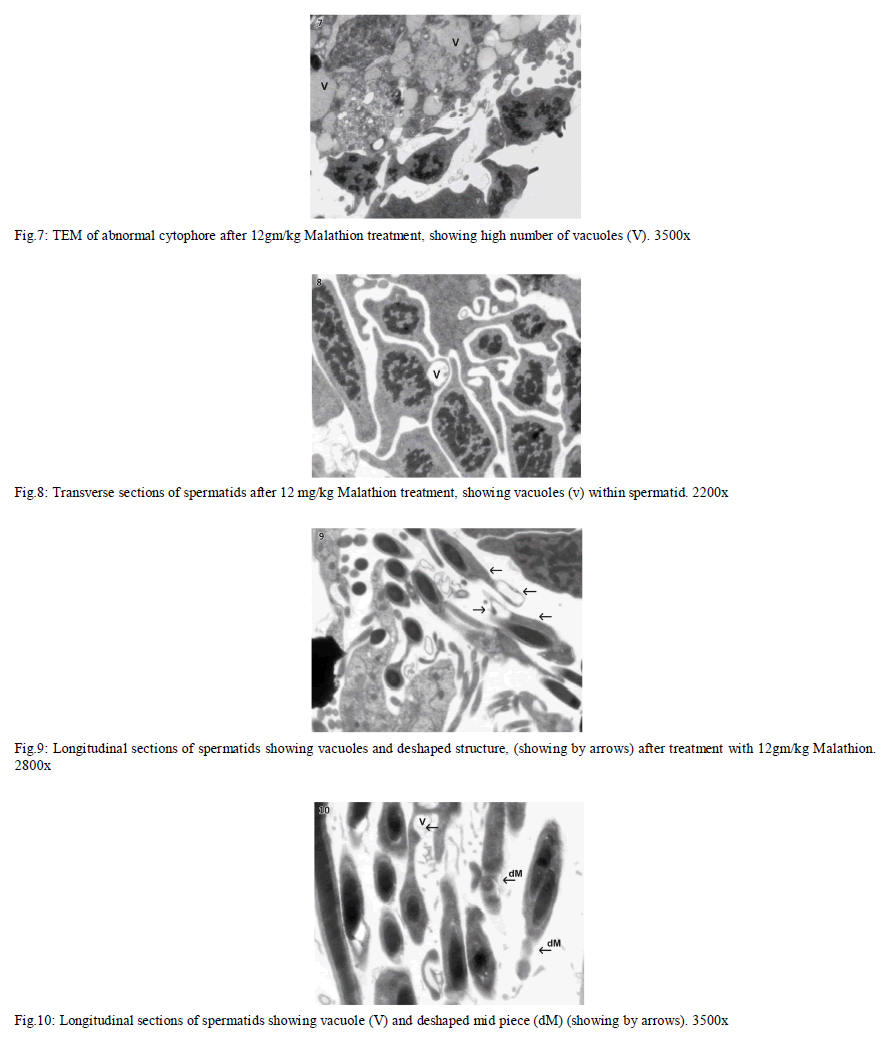 |
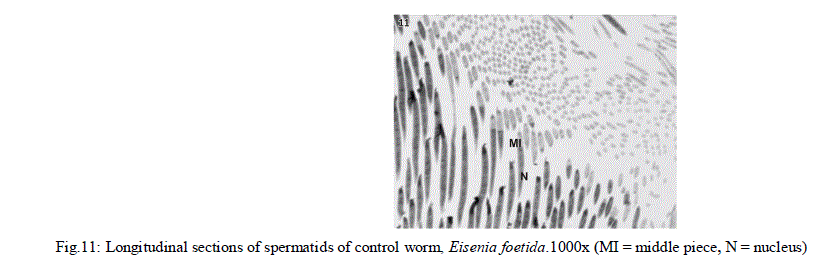 |
| Invertebrates are frequently used as biomarkers for the possible effects of xenobiotics applied to the environment [20]. Earthworms have been successfully used as sentinels of soil and also for non target toxicity tests of chemicals used in agricultural practices [21]. Currently, there is much concern about the adverse effects of environmental chemical agents in biological systems [22]. Malathion is lethal to many living systems due to its indiscriminate utilization in different fields like, agriculture, houses and gardens. Malathion affects the neuromuscular function, altering the balance of Ca++ and K+ which seems to be the factor responsible for changes in the morphology and activity of the earthworms. This would explain the phenomenon of coiling of the tail, observed in all individual treated with Malathion [18]. Previously many authors ([23], [24], and [25]) have performed a detailed study on spermatogenesis of Eisenia foetida including light and electron microscopy. These studies include information on the cytophore and gonial development. And later have been summarized by reference [26]. The present study gives concrete pictorial evidence that Malathion causes abnormality in the ultra structure of cytophore as well as affects spermatids of Eisenia foetida. These evidences are well supported by the references [27], [28], [29]. Other studies report that spermatozoa could be damaged by the effect of pesticide on spermatogonical morula, altering the ultra structure of the cytoskeleton of the cytophore (common cytoplasmic mass), affecting therefore the normal development of the spermatogenesis and the spermiogenesis [17]. Malathion has an acute and direct cytotoxic effect (cellular death) on human lymphocytes, without causing damage to the DNA, but their active metabolites, malaoxon and isomalathion, could act later by breaking DNA chains and Malathion degraded to its active metabolites malaoxon and isomalathion, which could also alter spermatogenesis ([18],[30]). According to references [31], [32], [33] and [18], E. foetida treated with Malathion exhibited a completely disordered structure with loss of central hillus, vacuolization and cells with small and pyknotics nuclei. This emphasizes the idea that Malathion alters the testicular function seriously in this species, as reported earlier for earthworms and mice treated with other organophosphates pesticides. Malathion has a direct cytotoxic effect causing coiling of the tail, with increase of metachromasia of the chromatin of the spermatozoa and altering the sperm count and also affects testicular structure, with increased vacuolization and hyperpyknosis of the nuclear of spermatogonia [18]. Damage to spermatozoa can be of particular importance since it may cause adverse effects that may not be "visible" in terms of the morphology of the organism or male fertility but in terms of fetal loss and congenital malformations ([34], [35]). According to ref. [36], in mice it has been reported that Malathion was a potent cell cycle inhibitor that induces DNA damage and is capable of interfering with DNA replication; this suggests that Malathion is a genotoxic agent and may be regarded or a potential germ cell mutagen. The main objective in the ecotoxicological work is to provide information to estimate ecological risk [37], but the relative inability to identify critical endpoints remains a fundamental problem. According to ref. [38], features to study in this regard are the biochemical, cellular and physiological effects. Our study evidently confirms that the information provided at the ultra cellular level by ultra structural changes during spermatogenesis in E. foetida could well in future fulfils the above criterion. It can be concluded from this electron microscopic investigation of male reproductive organ of E. foetida after exposure of Malathion that, it is a cytotoxic agent that alters reproductive capability of E. foetida and ultimately reduce the reproductive out put of this worm. |
References |
|