ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Sanjay Mavanuri1, Varsha Patil1, Manjunatha Hanumanthappa1, ShivarudrappaB.Bhairappanavar2 Sadashiv. S. O3., SanthoshKondajji Hanumanthappa1, Venkatarangaiah Krishna1, Chandrashekhar Unakal*4
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The methanolic leaf extract of BrugmansiasuaveolensBercht. &Presl.was subjected for scientific investigation to support its traditional use as wound healer. Wound healing activity was evaluated in different concentrations prepared as an ointment in sodium alginate using five groups of six Wistaralbino male rats in each model with povidone iodine (5%) as the standard drug. Wound healing was assessed by a rate of wound contraction, tensile strength and histological changes. The results demonstrate high rate of wound contraction, higher wound breaking strength in experimental animals treated with 50 mg kg-1 of methanolic extract as compared to control and standard povidone iodine. These findings were further confirmed by histological examination of granulation tissue with a lesser number of chronic inflammatory cells, decreased edema and increased collagenation than the corresponding controls. However, effectiveness of plant extract’s wound healing property needs to be further verified before being considered for clinical purpose.
Keywords |
| Brugmansiasuaveolens, leaves, methanolic extract, wound healing activity |
I. INTRODUCTION |
| Wound refers to the disruption/opening in the epithelial tissue of the skin, caused by physical, chemical, thermal and mechanical integrity and infection occurred by microbes present in the environment and delay the wound repair (Shuid, 2005).Wound repair is a natural process of reconstructing dermal and epidermal tissue. Injury to the tissue may result in cell death and tissue destruction and also involves continuous cell-cell and cell-substrate interactions that allow the process to progress in three overlapping phases viz. inflammation (0–3 days), cellular proliferation (3–12 days) and remodeling phases (3–6 months), respectively (Glynn, 1981; Clark, 1996; Martin, 1996). Plants are the store house of chemicals and act as potential healer. Many researchers reported on wound healing activity of indigenous medicinal plants in recent years (Sharathet al., 2010), (Kumar swamyet al., 2006) Portulaca oleraceaL. (Rashedet al., 2003), Tephrosiapurpurea(Marwah, et al., 2006) and Centaureasadleriana(Csuporet al., 2009).The plant BrugmansiasuaveolensBercht.&Presl of family Solanaceae is endemic to Malnad region (Shimoga ) of the Western Ghats, Karnataka, India. Traditionally leaf extract of B. suaveolenshas been mainly applied externally to treat cut wounds, swellings, scalds, inflammations, skin rashes and hemorrhoids. It also possesses spasmolytic, anti-asthmatic, anticholinergic, narcotic and anesthetic properties (RawiaZayed and Michael Wink, 2004). Analgesic effect of aqueous extract of B. suaveolensflower in mice was evaluated by Ana LuizaMuccillo-Baisch (2010). Phytochemical analysis reveals that the presence of bio-active compounds 1, 8-cineo, (E)-nerolidol and a-terpineol (Samuel et al., 2009). Literature survey reveals that no experimental study has been made to authenticate folklore wound-healing property of the leaves of B. suaveolens. In the present study an attempt has been made to investigate the wound healing activity in Wistar albino rats by excision, incision and dead space wound models. |
II. MATERIALS AND METHODS |
| Plant Material Collection and Preparation of Plant Extract |
| The plant material was collected during flowering stage in the month of October, 2010. Near HosanagarTaluk, ShimogaDist, Karnataka, India. Authentication has done by Prof. Y L Krishnamurthy and voucher specimen was deposited at Department of Applied Botany, Kuvempu University, Shankaraghatta, Shimoga, Karnataka, India. Voucher No: KU/AB/HS/1010. The shade dried plant material of B. suaveolenswas pulverized and subjected for sequential Soxhlet extraction using different solvents like petroleum ether, chloroform and methanol. The crude sample which was collected from the Soxhlet extraction is concentrated using rotary evaporator and these samples were stored in desiccators to avoid moisture absorption. |
| Experimental Animals |
| Wistar albino male rats of body wt. between 150-200g were used for wound healing activity and were procured from Venkateshwar Traders Bangalore. The rats were housed in standard environmental conditions, fed with standard food and water ad libitum during the whole period of the experiment. The approval of Institutional Ethical Committee (Reg.No:144/1999/CPCSEA/SMG) was obtained before execution of the study. |
| Acute Toxicity |
| Acute oral toxicity (Ecobichon, 1997) study was performed as per OECD-423 guidelines (acute oral toxic class method). Albino male rates (n = 6 in numbers) were used for the study. The animals were kept fasting for overnight providing only ad libitum water, after which the methanol extract was administered orally at the dose level of 1000, 2000 and 3000 mg kg-1body weight by intragastric tube and observed for 72 h. This dosage was gradually increased up to 5000 mg kg-1until any behavioral changes or mortality was observed. |
| Preparation of Drug Formulation |
| The drug formulations of methanol extract (30 mg kg-1 and 50 mg kg-1) of leaves of B. suaveolenswere prepared using white petroleum jelly and used for evaluation of wound healing activity along with (5%) povidone iodine (Kalyon Roy, 2009). For oral administration, suspension of 500 and 1000 mg/ ml extract was incorporated with Tween-80 (1% w/v). |
| Experimental Animals |
| Animals were divided in to four groups, each group consisting 6 rats. Group I: Received no treatment and served as control. Group II: Received application of standard drug ointment i.e. povidone iodine cream (5%). Group III: Received topical application of test sample 30 mg kg-1). Group IV: Received application of test sample (50 mg kg-1). |
| Evaluation of Wound Healing Activity |
| Excision, incision and dead space models were used to evaluate the wound healing activity of leaf methanol extract of B. suaveolens. |
| Excision Wound Model |
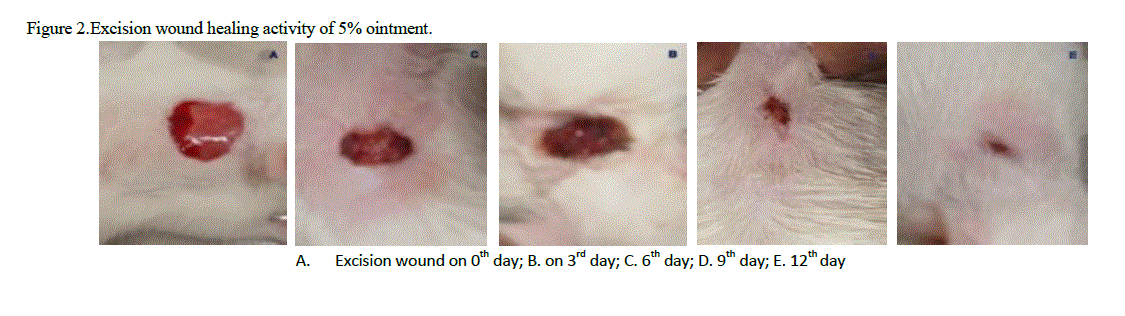
| The rats were inflicted with excision wound as described by Morton et al. (1972) under light ether anesthesia. Cutaneous circular wounds of 200mm2 were inflicted on the pre-shaved semi-sterile dorsal surface of thoracic region of rats with the help of sterilized surgical scissors and forceps under semi-aseptic condition. The skin of impressed area was excised to the full thickness to obtain 200mm2 width and 2mm depth. Each animal was housed individually in separate polypropylene cages. The drugs were topically applied daily until the formation of complete epithelial layer, starting from the first day of operation. The wounds were traced on graph paper on the day of 3rd, 6th, 9th and 12th. The wound closure was measured at regular intervals of time to monitor the percentage of wound closure and epithelialization time that indicates the formation of new epithelial tissue to cover the wound. The number of days required for falling of the scar without any residual of the raw wound gave the period of epithelialization. |
| Measurement of Wound Area |
| The observation of percentage of wound closure were made on 0th, 3rd, 6th, 9th, and 12th days of post wounding days. The area of wound was recorded by placing a transparent sheet over the wound and marked by a permanent marker. The wound area recorded was measured in mm2 by using graph paper. |
| Incision Wound Model |
| The rats were divided into 4 groups n = 6 in each groups. The animals were anesthized using light ether. Para vertebral incision of 5 cm was made on either side of vertebral column of rat with the help of sharp scalpel and incision was made at least 1 cm lateral to vertebral column with sufficient care. The incision was closed with interrupted sutures at a distance of 1 cm. These rats were housed individually in different cages for 10 days. The animals were daily treated with ointment from 1st day to 10th day. On the 11th day of post wounding, wound breaking strength was measured by adopting continuous constant water flow technique as described by Lee and Tong et al. (1968) and breaking strength was expressed as the minimum weight of water necessary to bring about the gapping of the area. |
| Dead Space Wound Model |
| For dead space wound model the animals were divided into three groups containing six in each. Group I served as the control that received 1ml kg-1of water orally. The remaining group II and group III were administered with oral dose of 30 and 50 mg kg-1of test substance respectively. The animals were anaesthetized by light ether and dead space wounds were created through a small transverse incision made in the lumbar region with sterilized cylindrical grass piths (2.5 × 0.5 cm) one on either side beneath the dorsal Para vertebral lumbar skin (Nayak, 2003). The day of the wound creation was considered as day zero. Granulation tissue formed on the grass pith was harvested by careful dissection on day 10th of post wounding day and subjected to breaking strength and histological studies. |
| Histological Study |
| The healing tissues obtained on the 11th day from all three groups of animals of the dead space wound model were processed for histological study to determine the pattern of lay-down for collagen. Ten percentage of neutral formalin solution was used to fix the granulation tissues for 24 h. and dehydrated with a sequence of ethanol-xylene series of solution. The inflicted material embedded with paraffin at 40-600 C were subjected for microtome section, stained with hematoxylin-eosin and observed under microscope for any histological changes. |
| Statistical Analysis |
| Statistical analysis was performed on each group (n=6), and an ANOVA test (using EZ ANOVA statistical software) was used to compare the mean values of each treatment. Significant differences between the means of parameters were determined by using the Dunnett’s T test (P < 0.05). The results represented means, standard error (SE) of six replicated determinations |
III. RESULTS |
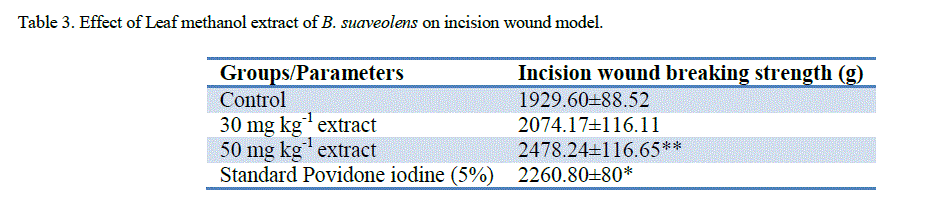
| ‘Staircase’ method was adopted for the determination of acute toxicity of leaf methanol extract through oral administration to healthy Swiss albino male rats weighing 150–200 g, after 72 h. the LD50 of methanol extract was found to be more than 5000mg kg-1. The therapeutic dose 500mg/ kg-1 (LD50) was calculated as 1/10th of the lethal dose for the purpose of wound healing investigation. Significant wound healing activity in groups with topical application of B. suaveolensleaf methanol extract 30 mg kg-1 has shown 22.7% wound area closure while 50 mg kg-1 had shown complete wound area closure. Similarly standard povidone iodine (5%) exhibited 29.3% wound healing. Leaf methanol extract 30 mg kg-1 has demonstrated significant reduction in the wound area compared to standard at the same concentration as shown in Table 1. The 50 mg kg-1 ointment of B. suaveolenstreated animals showed 100% wound closure (Excision wound model) in 12 days after the wound healing period, while 30 mg kg-1 ointment showed 88.1% wound healing and standard povidone iodine treated animals exhibited 90.0% of wound healing activity (Table 2). In incision wound model, B. suaveolens(50 mg kg-1) ointment treated animals showed significant increase in breaking strength (2478.2±116.6**) and 30 mg kg-1 ointment treated animals showed breaking strength of 2074.2±116.1. The significant wound breaking strength was observed in animals treated with standard drug povidone iodine 2260.8±80* but the effects seen to be more than the corresponding extract concentration and the results were shown in table S3. Histology of tissue obtained from the leaf methanol extract (500 mg kg-1) treated group showed significant increase in collagen deposition with mild macrophages, neutrophils and inflammatory cells. The control group did not show much collagenation, instead more number of macrophages, neutrophils, lymphocytes and inflammatory cells were observed. In this investigation, the results show that leaf methanol extract of B. suaveolenspromoted the wound healing activity in excision, incision and dead space wound models. |
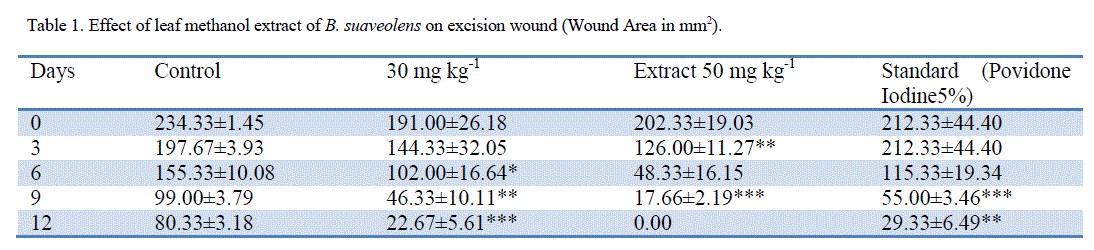 |
| Note: n =6 animals in each group, values are expressed as Mean ±SEM, If *=p<0.05, **=p<0.01,***=p<0.001 when compare to control. |
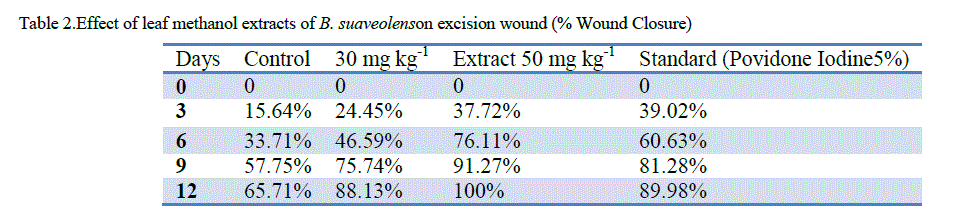 |
| Note: n =6 animals in each group, values are expressed as Mean ±SEM, If *=p<0.05, **=p<0.01, ***=p<0.001 when compare to control. |
 |
 |
 |
IV. DISCUSSION |
| Wound healing is a complex cellular process by which damaged cellular structures and tissue layers are restored to its normal stage. The length of healing process depends on the extent of injury and regenerating ability of the tissue. The aim of this study was to evaluate the wound healing potential of leaf methanol extract of Brugmansiasuaveolensusing Wistar albino male rats (150 to 200g) to determine wound healing activity created by mechanical injuries. The wound healing property of Brugmansiasuaveolensseemed to depend on preliminary phytochemical analysis of leaf methanol extract containing bioactive compounds like alkaloids, tri-terpenoids and flavonoids which were identified to promote the wound healing activity mainly because of their antimicrobial property, which seems to be responsible for wound contraction and increased rate of epithelialisation (Ya, 1988; Tsuchiya, 1996; Scortichini, 1991). The Volatile oils isolated from B. suaveolensflower were rich in β-ocimene, 1,8-cineole, with smaller amounts of phenethyl alcohol and nerolidol (Samuel, 2009) as this s-ocimene oil has shown strong inhibitory activity against gram Positive and gram negative bacteria and fungus (Aspergillusniger) (PeymanSalehia, 2006) while phenethyl alcohol acts as anti-Infective, antiseptic and preservative agent. The presence of nerolidol enhances or suppresses or changes the microbial mode of action (Hada, et al., 2003). In this report, the leaf methanol extract of B. suaveolenspromoted wound healing activity in excision, incision and dead space model. Wound healing is a complex process requiring the efforts of many different tissues and cell lineages during the phases of proliferation, migration, matrix synthesis and contraction. In excision wound healing model the 30 mg kg-1 ointment of leaf methanol extract treated animal group had shown a significant reduction in wound area and increase in period of epithelization as compared to standard (5%) povidone iodine treated and control group. Whereas, the 50 mg kg-1 ointment of leaf methanol extract of B. suaveolenstreated animal group showed more significant wound closer within 12 days as compared to the standard povidone iodine as this may be due to the presence of underlying chemical compounds like tannins, glycosides, saponin, alkaloids etc. (Evans, 1998) and polyphenols might exert wound healing activity by virtue of antimicrobial property (Kokate, 2008). Incision wound model had shown increased breaking strength and granulation tissue weight, which indicates the presence of higher protein content. Dead wound model was used to study the influence of extracts deemed to possess traditional ethno medical use for wound healing property on collagen deposition and granulation tissue. Histopathological studies revealed significant increase in collagen deposition and hydroxyl proline content in the dead wound space when compared to the control group of animals. The increase in collagen fibrils is responsible for the formation of skin, connective tissues (Singh et al., 2005) and strengthens extracellular matrix (Madden and Peacock, 1968). Collagen is the group of large proteins that prevents cell damage by foreign body’s and promoting the DNA synthesis, where the area of wound undergoes shrinking. Wound healing may be attributed to the phyto-constituents (phytosterols, glycosides, alkaloids, saponin, phenolic, Tannins, flavonoid and alkoloids) present in the plant and rapid closer of wound could be a function of either the individual or additive effect of phyto-constituents in the leaf methanol extract of B. suaveolens. |
V. CONCLUSION |
| Results demonstrate that the leaf methanol extracts of B. suaveolens would be capable of promoting wound-healing activity. However, it needs further evaluation in clinical settings before consideration for the treatment of wounds. Further studies with purified constituents are needed to understand the complete mechanism of wound healing activity of B. suaveolens. |
ACKNOWLEDGMENT |
| The authors acknowledged the help of UGC New Delhi for financial support. We thanks the Kuvempu University, Shimoga, Karnataka, India for providing the facilities and also thankful to the Principal, National College of Pharmacy, Shimoga for their kind support in conducting the experiments. |