ISSN ONLINE(2319-8753)PRINT(2347-6710)
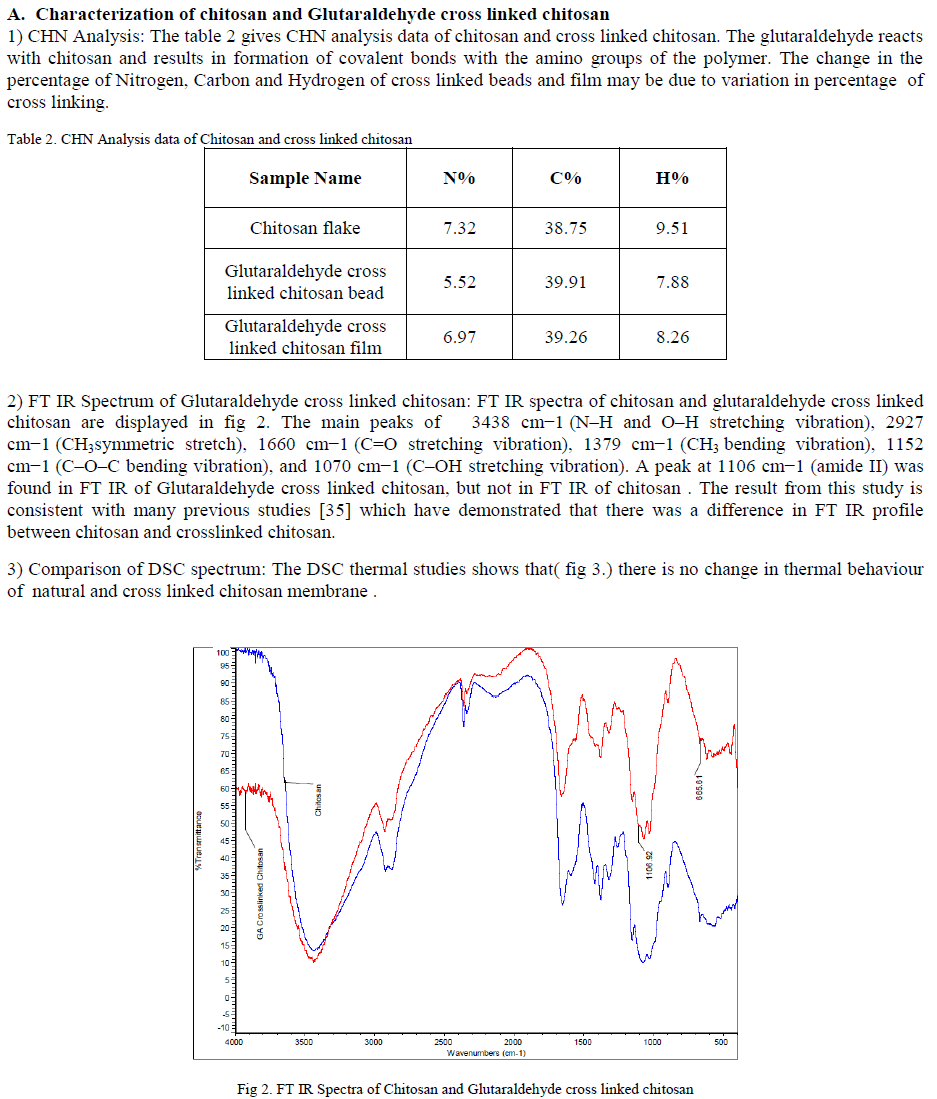
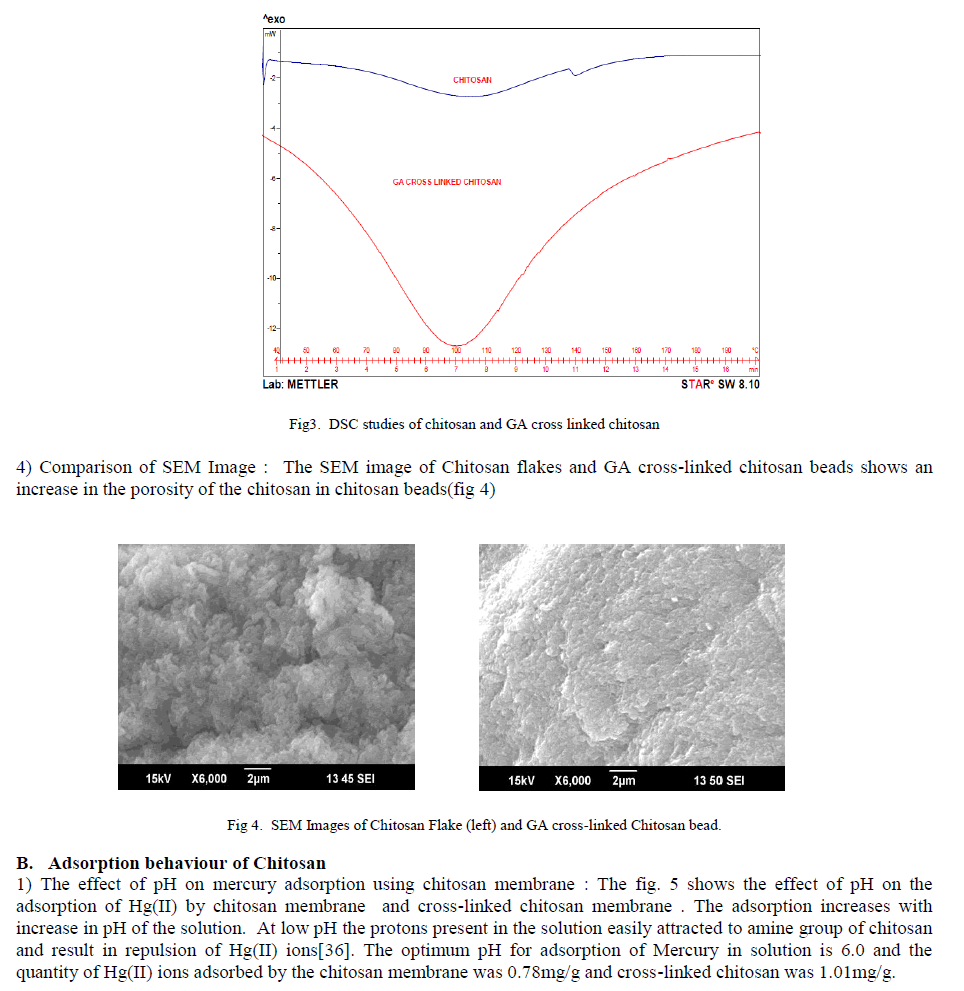
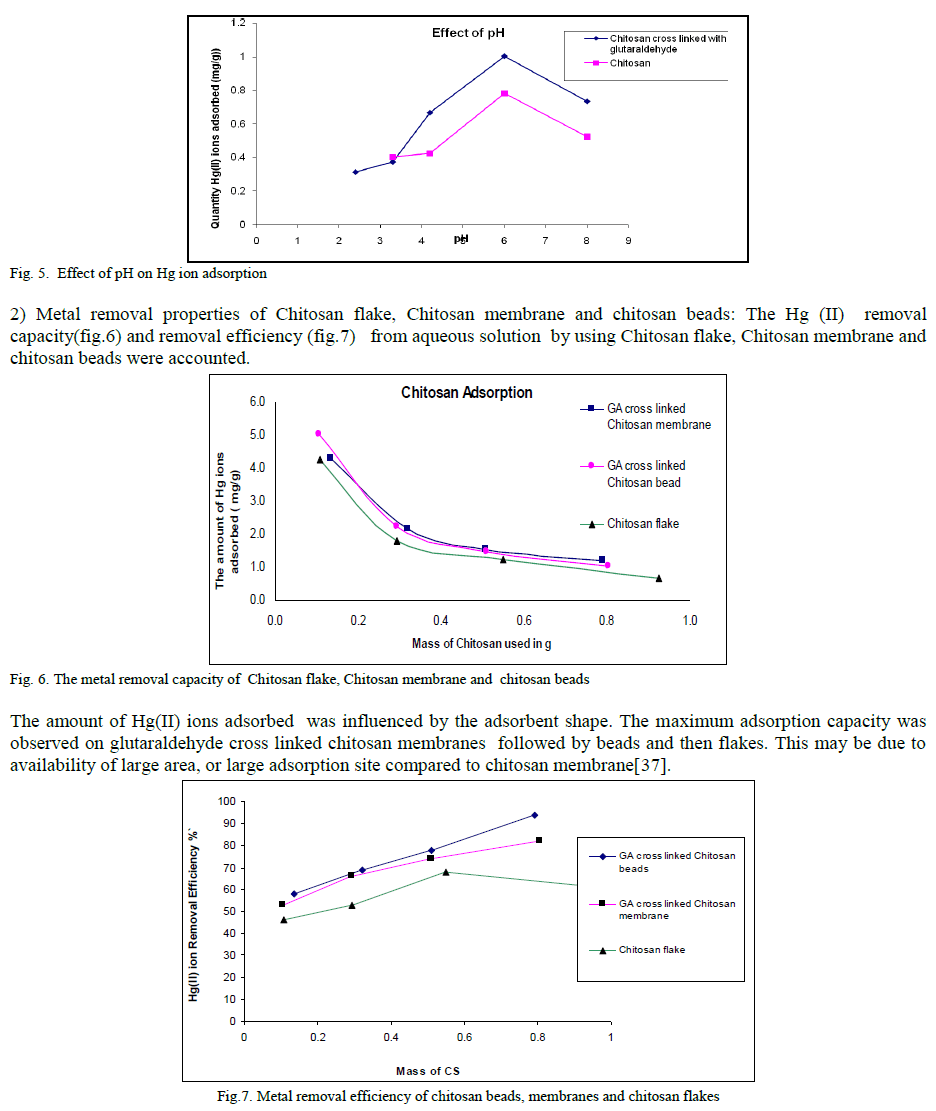
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Adarsh K J1*, Dr. G Madhu2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
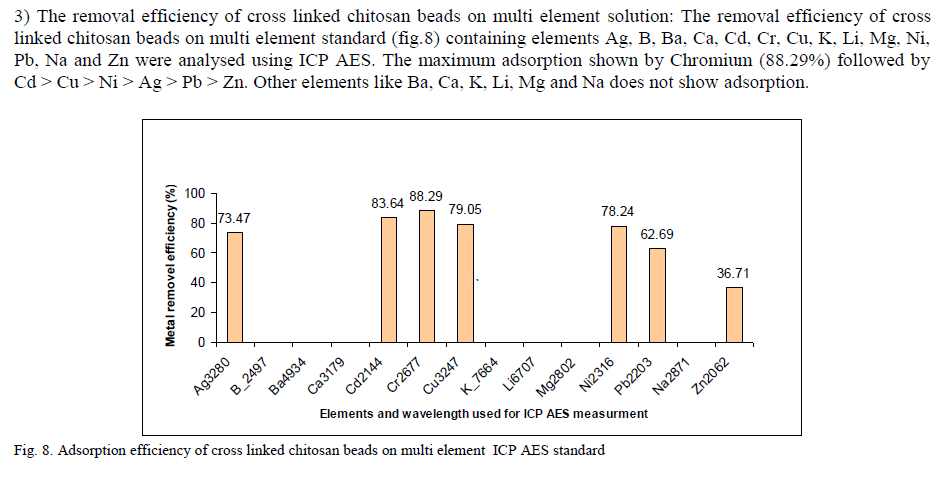
Application of chitinous products in waste water treatment has received considerable attention in recent years. The Chitosan, deacetylated chitin derivative is the most interesting biopolymer in the world of adsorption science.The preparation of chitosan beads and chitosan membranes were explained in this paper. The properties of chitosan flake, beads, and film was studied with different analytical method such as CHN, SEM, FTIR, DSC, etc. Chemical modification of chitosan membranes and beads were performed by crosslinking with glutaraldehyde and its properties were analysed.The Adsorption studies of Hg(II) ion was carried out in a batch process at room temperature in static manner with varying parameters such as pH of solution, adsorbent concentration and different Chitosan forms. Metal concentration was measured using ICP AES. It has been shown that cross linked chitosan beads have higher adsorption degree compared to chitosan flakes and cross linked chitosan membrane. The removal efficiency of cross linked chitosan beads on multi element solution shows that the maximum adsorption shown by Chromium (88.29%) followed by Cd > Cu > Ni > Ag > Pb > Zn
Keywords |
| Chitosan, Surface modification, Heavy metal removal, Crosslinking with glutaraldehyde |
Abbreviations |
| CS-Chitosan, GA- glutaraldehyde |
INTRODUCTION |
| The development of chitosan-based materials as useful adsorbent polymeric matrices is an expanding field in the area of adsorption science [1]. Chitosan is considered as answer to researchers interest in developing cost effective and environmental friendly technologies for the remediation of soil and water polluted with toxic trace elements. Its use as a biosorbent is justified due to following advantages, low cost compared to commercial activated carbon, ease in availability, removal efficiency of wide variety of metals and outstanding chelating behaviour [2]. The Chitosan, deacetylated chitin derivative is the most interesting biopolymer in the world of adsorption science [3-4]. Henri Braconnot, a French professor discovered chitin in 1811 [5] by first demonstrating the presence of acetyl groups in mushrooms [6] and in 1843 Lassaigne [7] unambiguously demonstrated the presence of nitrogen in chitin [8]. Henri Braconnot’s named chitin as fungine. In 1823, Odier[9] found the same material in insects and plants and named it chitine [10]. But he did not actually demonstrate the chitin identity. Payen (1843)[11] prepared and easily replicated chitin in the laboratory. Muzzarelli [12] has chronologically enlisted chitin and chitosan in the book “Chitin”. Chitin is the second most abundant polymer in nature after cellulose. Chitin is white, hard, inelastic, nitrogenous mucopolysaccharide consisting of a poly-beta-1, 4-linked N-acetyl glucosamine. Chitin is structurally similar to cellulose, but it is an amino polymer and has acetamide groups at the C-2 positions in place of the hydroxyl groups. The presence of these groups is highly advantageous, providing distinctive adsorption functions and modification reactions. The raw polymer is commercially extracted from marine crustaceans primarily because a large amount of waste is available as a by-product of food processing [15]. Chitin is the main component of arthropod exoskeletons, tendons, and the linings of their respiratory, excretory, and digestive systems [14]. It is also found in the reflective material (iridophores) both in the epidermis and the eyes of arthropods and cephalopods and also a component of the cell wall of fungi. There are three forms of chitin: α, β, and γ chitin. The α-form, which is mainly obtained from rab and shrimp shells, is widely distributed. Both α and β chitin/chitosan are commercially available. The α-chitin chains are aligned in antiparallel fashion. The anti-parallel arrangement in α-chitin give rise to strong hydrogen bonding and consequently makes it more stable [15]. The β-form mainly obtained from molluscs such as squids arranged parallelly, whereas the γ-form contains two parallel and one anti-parallel strands of chitin [16]. Chitin is insoluble in water due to its intermolecular hydrogen bonds [17]. The most mportant feature is its flexibility to convert into different forms such as fibres, hydrogels, beads, sponges, and membranes [18]. Chitosan is N-deacetylated derivative of chitin. A sharp nomenclature with respect to the degree of N-deacetylation has not been defined between chitin and chitosan [19]. The commercially available Chitosan is normally polymers with less than 20- 25% acetyl content. Chitosan and chitin are of commercial interest due to their high percentage of nitrogen compared to synthetically substituted cellulose (Fig.1 ). Chitosan is soluble in acid solutions and is chemically more versatile than chitin or cellulose. Chitosan has three types of reactive functional groups, an amino group as well as both primary and secondary hydroxyl groups at the C-2, C-3 and C-6 positions respectively .Its advantage over other polysaccharides is that its chemical structure allows specific modifications especially –NH2 group in the C-2 position. |
 |
| Chitin and chitosan exhibit special characteristics such as hydrophilicity, biocompatibility, biodegradability, nontoxicity, adsorption properties, film-forming ability, bio-adhesivity, poly-functionality etc. [20-21]. Chitosan can be used as an adsorbent to remove heavy metals due to the presence of amino(-NH2) and hydroxyl (-OH) groups, which can serve as the active sites [22]. However, chitosan is very sensitive to pH and other factors like; the origin and nature of the chitosan, the activation conditions of the raw polymer, chemical modifications, physical conditions (Temperature, stirring rate etc) and the influence of process variables (contact time, initial concentration, polymer dosage etc) Chitosan properties, such as aqueous solubility in neutral to basic media and mechanical characteristics, can be enhanced by chitosan modification. The –NH2 in the C-2 position allow direct substitution reactions and chemical modification in aqueous solution under mild conditions [23]. Chitosan cross-linking with glutaraldehyde(GA) is a widely applied method of surface modification. It will stabilize chitosan in acid solutions and enhance its mechanical properties [24]. It also increases its resistance to biochemical and microbial degradation. Moreover, chemical grafting of new functional sites improves extraction selectivity as well as maximum adsorption capacities and pH sensitivity [25]. |
| Different structural conditions of chitosan 1) Flakes: Most of the studies on chitosan have been performed on chitosan flakes, since they are easier to manage than gelled materials[34]. The major limiting parameter with flaked material will be the size of chitosan particles due to diffusion restrictions. 2) Gel beads: Gel beads has been widely used for the preparation of catalyst particles [26-27]. The preparation of the chitosan gel beads can be operated independently for the preparation of the catalyst. The polymer is dissolved in acetic acid and the viscous solution of chitosan is pumped through a thin nozzle into an alkaline coagulation bath [28- 29]. 3) Fibres & hollow fibres: For the preparation of fibres and hollow fibres, a two-step procedure is used (a) dissolving the polymer in acetic acid solution followed by (b) extrusion of the viscous solution through a thin nozzle and coagulation of the solution with a suitable neutralization or coagulation agent [30]. 4) Membranes: The chitosan can also be prepared as membranes. The limitations of chitosan membrane can be overcome with [31] an inorganic supports. 5) Inorganic immobilized support for chitosan: In many applications, the poor diffusion properties or weak mechanical properties (especially for thin membranes) necessitates the chitosan to be deposited in thin layers on the surface of porous materials to improve the specific surface area and mechanical properties and to optimize efficacy and accessibility of the chitosan’s internal amino groups. Silica beads, glass beads[32], perlite[33] are some of the immoblized supports used for chitosan. |
II.EXPERIMENTAL |
| A. Materials and methods A detailed study of Chitosan cross linking with glutaraldehyde and adsorption properties were carried out using chitosan flake, GA cross linked chitosan beads and chitosan membrane. Chitosan flakes with deacetylation degree of about 94% was collected from India Sea Foods, Cochin, India. The glutaraldehyde (50%) was purchased from Sigma Aldridge, India. All other chemicals and reagents used were analytical grade and used as received. The C-H-N analyzer (Elementar, Vario EL III) is used for elemental analysis of chitosan flakes and Cross linked chitosan membrane and beads. The cross linking of glutaraldehyde with chitosan was characterised by FT-IR spectrometer (Thermo Nicolet, Avatar 370 with HATR Assembly). Porous nature of chitosan and cross linked chitosan were compared with SEM Image (JEOL, JSM - 6390LV). Thermal properties are studied again by Differential Scanning Calorimetry (Mettler Toledo). The metal concentration was analyzed using ICP AES system (ThermoElectron, IRIS intrepid II).[34] B. Preparation of chitosan membranes Chitosan solution was prepared by dissolving 5g of chitosan flakes into 200 ml of 3% (v/v)acetic acid solution. Chitosan solution 2.5% (w/w) was spread on a Petri dish. The dish was kept at 60◦C, until a reduction of 50% in its initial weight was reached and kept one day at room temperature to form the membrane by natural drying. The membranes were then immersed in NaOH solution (1 M), for 24 hrs to neutralize the excess acid. The chitosan membranes were rinsed in Milli-Q water, air dried and stored. C. Preparation of chitosan beads The Chitosan solution 2.5% (w/w) was sprayed into a precipitation bath containing 250 ml of 1 M NaOH, which neutralized the acetic acid within the chitosan gel and thereby coagulated the chitosan gel to spherical uniform chitosan gel beads. The aqueous NaOH solution was stirred using magnetic stirrer. The wet chitosan gel beads were extensively rinsed with distilled water to remove any NaOH, filtered and finally air dried to remove the water from the pore structure [34]. D. Chemical modification The chitosan membrane was heterogeneously cross-linked in 0.25% (w/w) and 0.75% (w/w) aqueous glutaraldehyde solution (3.0 g of wet chitosan membrane in 50mL of glutaraldehyde solution) without agitation, at room temperature for 2 hrs. After 2 hrs cross linked chitosan membranes were washed in Milli-Q water to remove the unseated glutaraldehyde residues, air dried and kept in a closed container. |
| The chitosan beads were heterogeneously crosslinked in 0.75% (w/w) aqueous glutaraldehyde solution without agitation, at room temperature for 2 hrs. After 2 hrs cross linked chitosan beads washed in deionized water to remove the unseated glutaraldehyde residues , air dried and kept in a closed container. E. Bach Adsorption study 1) Mercury adsorption using chitosan membrane Batch adsorption experiments were performed to study the effectiveness of Chitosan membrane in the removal of Hg(II) ions from water. Effect of various parameters such as pH, Influence of chitosan dosage, glutaraldehyde cross linking was also studied. Standard stock solution of Hg (1000mg/L concentration) was diluted to 4mg/L and 10mg/L using Milli-Q water. The effect of Hg(II) ions adsorption was studied in the pH range of about 2–8. The initial pH of the solution was adjusted using 0.1 M HNO3 or 0.1 M NaOH. 0.2 g of chitosan membrane and GA cross linked chitosan membrane adsorbates were added to 50 ml of Hg(II) solution (4mg/L concentration) and equilibrated with magnetic stirrer for 24 hrs, at room temperature in a closed container. The membrane is removed and the metal concentration on the solution was measured using ICP AES. 2) Comparison of metal adsorption of chitosan flake, membrane and beads : The adsorption experiment by chitosan membrane, chitosan beads and chitosan flakes was carried out and the closed container 3) Multielement removal efficiency of cross linked chitosan beads Removal efficiency of cross linked chitosan beads was checked using multielement standard (elements Ag, B, Ba, Ca, Cd, Cr, Cu, K, Li, Mg, Ni, Pb and Na ions in solution) and ICP AES standard (Merck , Germany ). The 1000mg/L stock solution was diluted to5mg/L solution using Mill-Q water. 1g of chitosan beads was added to 100 ml solution and kept for 24hrs. The magnetic stirrer used to enhance the adsorption. The pH of the solution kept in the range of 5.1 to 5.4 using 0.1M HNO3 or 0.1M NaOH Solution. Samples are filtered using Whatman No.1 filter paper . The concentrationof metal ions present in the solution was analyzed using ICP AES. The adsorption capacity of chitosan membrane and beads were calculated based on difference of metal ion concentration in the bulk of solution before and after adsorption, as depicted in |
 |
III. RESULTS AND DISCUSSIONS |
| The physical parameters of the chitosan are given in the table1. The viscosity of the chitosan was measured using Brookfield Viscometer and Muzzarelli’s spectroscopy method used for Degree of acetylation (DA) measurement. Table .1. Physical parameters of the Chitosan used for analysis. |
 |
 |
 |
 |
 |
IV. CONCLUSION |
| The Chitosan and cross linked chitosan characterization studies were carried out to establish the previously discussed properties of chitosan .Metal adsorption capacity and efficiency of cross linked beads are higher compared to chitosan flakes and cross linked chitosan membrane. The optimum pH for adsorption of Hg(II) by Chitosan and GA cross linked chitosan is 6. The degree of adsorption varies with chitosan mass, pH of the solution, form of chitosan, cross linking degree etc. Glutaraldehyde cross linked chitosan have a tremendous capability in accumulating Ag2+ , Cd 2+ , Cu 2+ , Cr 2+, Pb 2+ , Zn2+, Hg2+ and Ni 2+ from the aqueous solutions. |
ACKNOWLEDGEMENT |
| All experiments were performed at Sophisticated Analytical Instrument facility (SAIF) at STIC, Cochin. The author is thankful to The Director and staff of STIC for their support. |