ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Rafael de Castro Ferreira1, Cláudia Rossetti1, David Feder1, Ligia Ajaime Azzalis2,Fabio Ferreira Perazzo2,
Virginia Berlanga Campos Junqueira2, Edimar Cristiano Pereira2, Paschoal Rossetti Filho3, Fernando Luiz
Affonso Fonseca1,2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Iontophoresis is a non-invasive technique that uses an externally applied small electrical potential difference to provide a controlled way to increase the transdermal transference of a variety of active agents with poor absorption/permeation through the skin. The aims of this research were (1) to determine if the inversion of phase was indicated during the performance of the iontophoresis; (2) standardize the time for ionization and (3) to propose the adequate frequency and polarity to reach different skin layers. Observing the depth and intensity of China ink (pH 7.8 + 0.2) transferred through the layers of ex vivo human tissues from the abdominal area, it was possible to show that the inversion of phases was not a viable methodology to increase the permeation of active agents, and it could even decrease the quality of permeation of a conduction that started correctly.
Keywords |
| Delivery/vectorization/penetration, Skin barrier, Skin physiology/structure. |
INTRODUCTION |
| The skin has been investigated as a route of drug administration and many techniques are been used to facilitate permeation of drugs across this organ [1]. Among these, iontophoresis is one of the most promising drug delivery system [2]. Iontophoresis is a non-invasive technique that uses an externally applied small electrical potential difference (0.5 mA/cm² or less) to provide a controlled way to increase the transdermal transference of a variety of active agents with poor absorption/permeation through the skin [3]. |
| Many characteristics of iontophoresis must be controlled to achieve drug delivery. Physicochemical properties of the drug (pKa, solubility), formulation composition (pH, drug and enhancers concentrations, ionic strength), current density level, and differences in skin types should be evaluated for each drug/enhancer combination [3, 4]. |
RELATED WORK |
| To which a drug is delivered by iontophoresis is uncertain. Most studies have demonstrated penetration to a depth of 3 to 20 mm [5, 6]. The iontophoresis parameters vary in clinical trials, affecting the ability to standardize treatments. Therefore, it is important to determine which component, either time or current amplitude, has a greater effect on drug absorption [7]. Moreover, the inversion of phase throughout the iontotherapeutic procedure is a common practice in Brazil in order to increase permeation. Based on the data supplied by the literature, the viability of this type of therapeutic conduct has been questioned. |
PRESENTATION OF THE MAIN CONTRIBUTION OF THE PAPER |
| The aims of this study were (1) to determine if the inversion of phase was indicated during the performance of the iontophoresis; (2) standardize the time for ionization and (3) to propose the adequate frequency and polarity to reach different skin layers. |
PROPOSED METHODOLOGY |
| All experiments were conducted according to the protocol approved by the Ethics Committee of Faculdade de Medicina do ABC. In order to avoid the use of animals, studies were performed ex vivo in human tissues from the abdominal area supplied by plastic surgery procedures. |
| The iontophoresis was conducted using a galvanic micro-current device (Physiotonus Microcurrent, Bioset®). H2SO4 0.02 mol/L was used as the anodic donor solution [8] whereas the 3% (v/v) pilocarpine was used as the cathodic donor solution [9]. |
| The tests in order to observe the inversion of phase were conducted as follows: |
| 1st conduction: 0.5 mL of China ink (pH 7.8 + 0.2) and 0.5 mL of H2SO4 at the positive pole; 1 mL of pilocarpine at the negative pole. The electric current was 200 μA/ cm2 for 20 minutes. |
| 2nd conduction: 1 mL of H2SO4 at the positive pole; 0.5 mL of pilocarpine and 0.5 mL of China ink at the negative pole. The electric current was 200 μA/ cm2 for 20 minutes. |
| 3rd conduction: 0.5 mL of China ink and 0.5 mL of H2SO4 at the positive pole; 1 mL of pilocarpine at the negative pole. The electric current was 200 μA/ cm2 for 10 minutes. The polarity was inverted and the test was carried out for 10 minutes more. |
| 4th conduction: 1 mL of H2SO4 at the positive pole; 0.5 mL of pilocarpine plus 0.5 mL of China ink at the negative pole. The electric current was 200 μA/ cm2 for 10 minutes. The polarity was inverted and the test was carried out for 10 minutes more. |
| 5th conduction: 0.7 mL of China ink, pH 4.0 + 0.5 (adjusted with 0.2 mL of buffer solution, pH 4.0) and 0.5 mL of pilocarpine at the negative pole; 1 mL of H2SO4 at the positive pole. The electric current was 200 μA/ cm2 for 20 minutes. |
| Control group: 1mL of China ink remained in contact with the skin for 20 minutes. |
| To standardize the time for ionization and to propose the adequate frequency and polarity to reach different skin layers, 1 mL of H2SO4 was placed at the positive pole, 0.5 mL of pilocarpine + 0.5 mL of China ink were used in the negative pole and electric current was 200 μA/cm2 for 20 (group 1), 15 (group 2), 10 (group 3) or 5 (group 4) minutes. |
| After the treatments, the skins were collected and fixed with 10% phosphate buffered formalin. Graded dehydration, paraffinization, and embedding were carried out on the fixed specimens. Paraffin sections were cut and processed for hematoxylin-eosin (HE) or picrosirius red (HP) staining for image analysis [10]. The images were captured with a NIKON ECLIPSE E800 microscope and the Micrometrics Plus software. |
| All tests were performed in triplicate and according to the Good Laboratory Practices. |
| Images were evaluated by two independent pathologists who were blind to the experiments. The correct indication of the applied current was validated by quantifying the intensity of incidence of China ink in the tissues with the + symbol. Once the intensity of the dye in the deepest layer was correlated, the non-ionized control was used as a higher intensity pattern in the other conduction ratings. After that, the depth the dye reached in relation to the procedure time was evaluated. |
EXPERIMENTAL RESULTS AND DISCUSSION |
| Table 1 shows that the inversion of phase was not a viable methodology to increase the permeation by iontophoresis. |
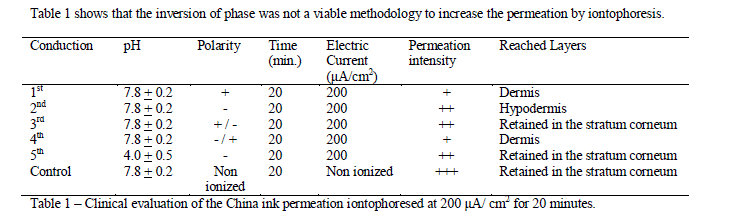 |
| It is known that the China ink is an alkaline substance, and thus it is attracted to negative charges. When added in an acid medium, it does not present a high permeation capacity even after 20 minutes of conduction. A small amount of the dye could be found on the dermis, close to the glandular area, and a large amount was still retained on the stratum corneum (Figure 1). |
 |
| Figure 1: 1st. China ink (pH 7.8 ± 0.2) conduction at the positive pole at 200 μA/ cm2 for 20 minutes, 40 X ocular magnification, HE stained slide. The dye was partly retained in the stratum corneum and partly permeated in the glandular area. |
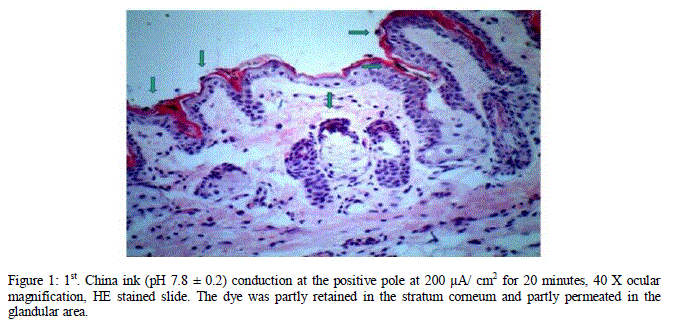
| The induction of an alkaline substance, a generator of negative charges at the negative pole, enhances the electrorepulsion effect by conducting a larger amount of the dye to deeper skin layers, a fact stated in the literature and shown in figure 2. In this figure a large amount of the dye can be seen in the deep hypodermis and close to a blood vessel. |
 |
| Figure 2: 2nd. China ink (pH 7.8 ± 0.2) conduction at the negative pole at 200 μA/ cm2 for 20 minutes, 40 X ocular magnification. The slice was stained with Picrosirius Red. It permeated the hypodermis deeply. Concentrations of the dye can be seen accumulated by a blood vessel. |
| The 3rd and 4th conductions evaluated the phase inversion in the iontophoretic conduction, a widely indicated method observed in the literature. It is important to point out that this technic was raised to prevent the skin polarization condition during the application of the direct current and thus avoiding lesion risks. Therefore, the inversion is justified for skin protection purposes only [11]. |
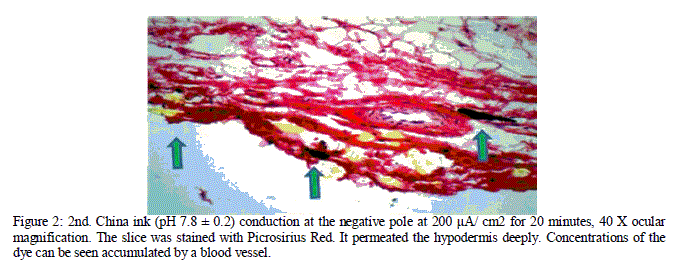
| The results showed that the phase inversion was not advantageous in the induction of active agents through the skin. In the 3rd conduction, although the China ink was added to an acid medium, where, theoretically, the formation of ions would be favorable, the permeation was hindered. In the 4th conduction, the dye was added to an alkaline medium, but the phase alteration decreased skin permeation significantly as shown in figures 3 and 4. |
 |
| Figure 3: 3rd. China ink (pH 7.8 ± 0.2) conduction with inversion of phases, beginning at the positive pole at 200 μA/ cm2 for 10 minutes, and then at the negative pole at 200 μA/ cm2 for 10 minutes. 40 X ocular magnification. The slice was stained with HE. Concentrations of the dye can be seen retained on the stratum corneum. |
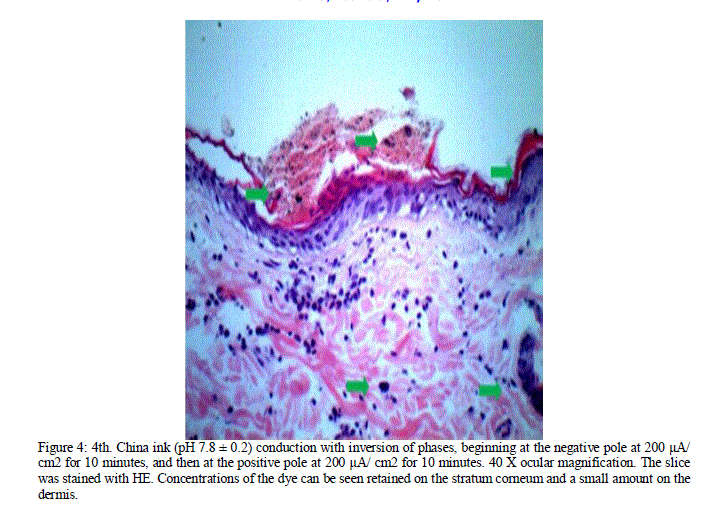 |
| Figure 4: 4th. China ink (pH 7.8 ± 0.2) conduction with inversion of phases, beginning at the negative pole at 200 μA/ cm2 for 10 minutes, and then at the positive pole at 200 μA/ cm2 for 10 minutes. 40 X ocular magnification. The slice was stained with HE. Concentrations of the dye can be seen retained on the stratum corneum and a small amount on the dermis. |
| These data reinforce the idea of the electroosmotic mechanism, i.e., when a substance is added to an alkaline solution with the same pH profile, there is still a fraction of active agent being ionized. The non-ionized fraction will be favored by the conduction at the pole of the correct formation of electric current. Moreover, there is an increase of cutaneous bioavailability of active agents when they are conveyed in an alkaline solution with the same pH profile of the area which application is targeted to. |
| The literature does not indicate which of the iontophoretic mechanisms of action is of greater importance, but according to the results obtained, a synergism of actions, resulting in higher permeation and greater depth can be observed. The proposal of vehiculating active agents in alkaline solutions with pH profiles different from theirs is questionable. The literature review indicates that anions, cations and neutral substances of low or high molecular weight are transferred by iontophoresis more effectively if compared with the amounts transferred by passive diffusion not influenced by the passage of an electric current. However, our results indicate a synergism of activity in active agents, vehiculated in alkaline solutions of the same pH profile and an extreme reduction in the permeation of active agents, vehiculated in a base solution of opposite profile, as shown in figure 5. |
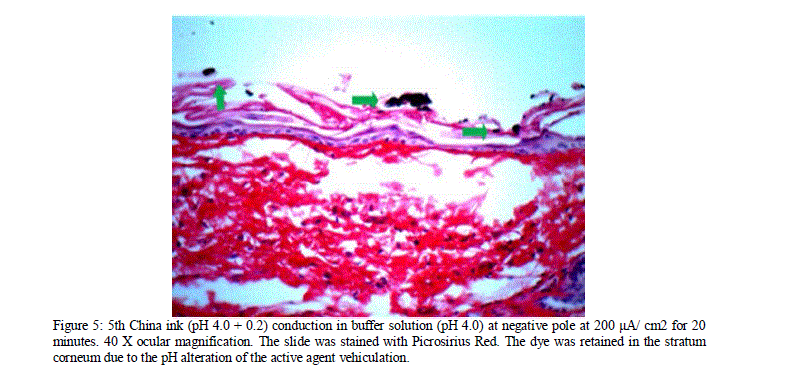 |
| Figure 5: 5th China ink (pH 4.0 + 0.2) conduction in buffer solution (pH 4.0) at negative pole at 200 μA/ cm2 for 20 minutes. 40 X ocular magnification. The slide was stained with Picrosirius Red. The dye was retained in the stratum corneum due to the pH alteration of the active agent vehiculation. |
| It is important to remark that a small concentration of active agents may permeate the tissue, even in base with an opposite pH profile due to the increase of the skin’s intrinsic permeability when the electric flux is applied. Upon developing a formulation should not be overcharged with many ionizable active agents because the ion association in the same solvent seems to be disadvantageous. Higher relative amounts of co-ions in the donor solution will compete with the drug ions through the transference [12]. |
| After the previous results, the dye at the negative pole in a solution with pH similar to its profile was standardized. The results obtained in this kind of conduction were the following: the longer was the conduction time, the deeper were the reached skin layers, as shown in the table 2. |
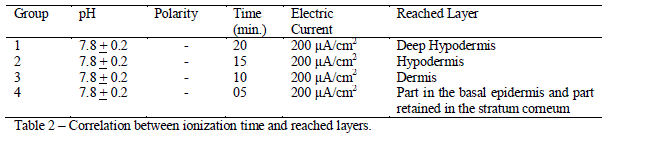 |
| This last procedure was performed as a standardization suggestion for time of conduction according to each type of treatment. Group 1 presented a very deep permeation. Patients who do not have thick layers of adipose tissue should be ionized for 15 minutes. Otherwise, the ionization time should be between 15 and 20 minutes. For those treatments which target the dermis, the suggestion time would be at least 5 minutes, in areas where the skin is thin, and 10 minutes in the other areas. For treatments which target the epidermis, the conduction should not exceed 5 minutes. |
CONCLUSION |
| The results showed that the inversion of phases was not a viable methodology to increase the permeation of active agents, and it could even decrease the quality of permeation of a conduction that started correctly. |
| Acid and alkaline active agents should be vehiculated in bases of their same profile for the synergism of the electrorepusion and electroosmosis and for facilitating the increase of the skin permeation through an electric flux application. |
| Results indicate that the formulations should be developed with active agents and bases that present the same pH and charge generation profiles. Moreover, they also suggest that the overcharge of ionizable active agents in a formulation is harmful due to the ionic competition in the conduction. Such formulations should also have the pH level closer to the level of the application area so that the cutaneous bioavailability of the active agent is favored. |
| Ionization time is paramount in the chosen of the treatment. Individually adapted to patients, each treatment proposes different ionization times, i.e., a treatment that targets the hypodermis should be ionized in a time interval between 15 and 20 minutes, the dermis, between 5 and 10 minutes, and a treatment that targets the epidermis should not exceed 5 minutes of ionization. |
| Authors’ contributions: RFC, CR and PRF contributed to analysis and interpretation of data. DF performed the statistical analysis. LAA drafted the manuscript. FFP, VBCJ and ECP revised the manuscript critically. FLAF conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript. |
| Competing Interests Statement: The authors declare no competing interests. |
References |
|