ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Shivam Bajpai1, Sundaram Singh2 and Vandana srivastava3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The reaction of isatin and N-substituted isatins with o-phenylenediamine readily gives rise to quinoxaline and spiro-indole derivatives respectively under controlled microwave irradiation in xylene. The results obtained under microwave assisted reaction have been compared with those obtained under conventional method. The chemical structures of the respective synthesized compounds were confirmed by analytical and spectral data.
Keywords |
| N-substituted isatins, quinoxaline derivatives, spiro-indoles, xylene, microwave irradiation. |
INTRODUCTION |
| The discovery of novel synthetic methodologies to facilitate the preparation of the compound libraries is a pivotal focal point of research activity in the field of modern medicinal and combinatorial chemistry [1]-[2]. Our approach to address this challenge involves the development of microwave assisted reaction for the quick synthesis of compounds. Microwave–induced organic reaction enhancements (MORE) chemistry can also be expressed e-chemistry due to its effective, economical nature, easy to handle and it is a step toward green synthesis [3]-[5]. The Chemistry of quinoxaline and spiro-indole derivatives has received considerable attention owing to their synthetic and biological importance. Quinoxaline derivatives exhibit potent antiviral [6] & antibacterial properties [7]. Spirocyclic systems containing one carbon atom common to two rings are also structurally interesting [8] because they represent an important class of naturally occurring substances characteristic by their highly pronounced biological properties [9],[10]. Functionalized spirocycloalkyloxindoles and quinoxaline derivatives are found in a variety of natural products and bioactive molecules [11]-[13]. Isatin and substituted isatins have been extensively explored for developing pharmaceutically important molecules [14]. Functionalized N-substituted isatins are also reported to show different biological activities, such as antibacterial [15], anti-fungal [16], [17], antiviral [18], anti-HIV [19], [20] and antileukemia [21]. Since the original report of Schunck [22] the reactivity of isatins with amines has been extensively studied. [23]- [27] However, it was interesting for us to develop a new access to this class of compounds because of their pharmacological properties and our interest in the area of green synthesis [28], [29] prompted us to explore the use of microwave (MW) irradiation as an alternative energy source for the synthesis of quinoxaline and spiro-indole derivatives by the reaction of isatin and N-substituted isatin derivatives with o-phenylenediamine in xylene (Scheme 2). Xylene has high boiling point and can withstand the conditions of the reaction (high heat). The reactants are more soluble in xylene than the products, so the product is easily crystallized out of the solvent at the end of the reaction. |
RESULTS AND DISCUSSION |
| In view of biological significance of isatin moieties, it was planned to condense isatin moiety with o-phenylenediamine in xylene to get spiro-indole and quinoxaline derivatives via two step process. |
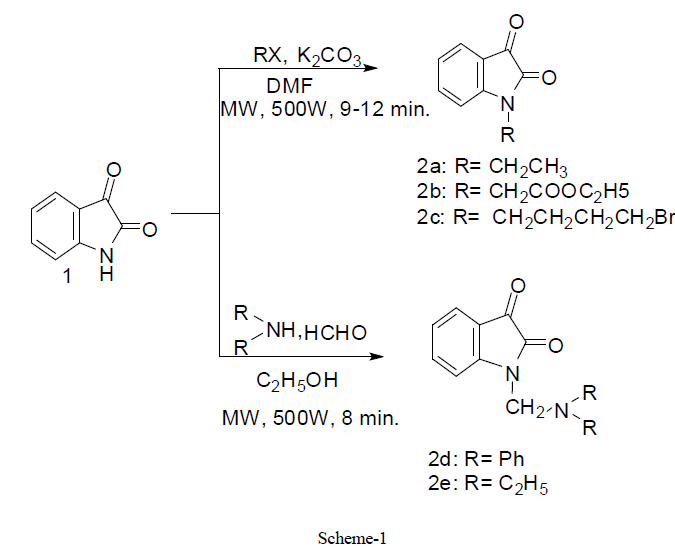 |
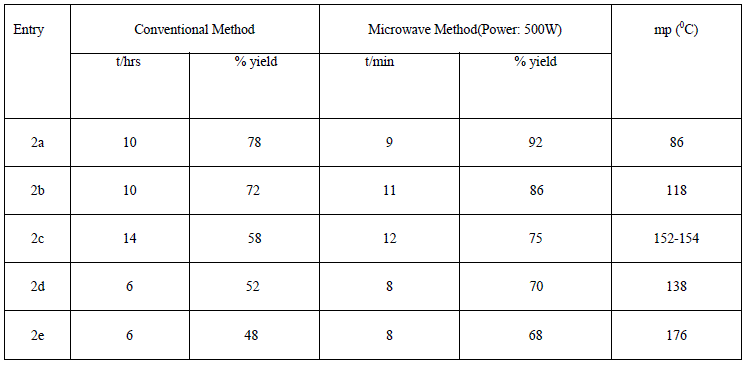
| In first step, we have synthesized N- substituted derivatives of isatin moiety by conventional method (48- 77.9%) and also under controlled microwave irradiation (68-92 %) (scheme1). All these compounds 2a-2e were synthesized by stirring of reaction mixture at room temperature in low yield (conventional method) whereas similar compounds were synthesized under microwave irradiation at 500W in good yield within 8-12 min. Actually, microwave irradiation facilitates the polarization of the molecules under irradiation causing rapid reaction to occur. The impact of microwave irradiation and conventional stirring for the synthesis of compound 2a-2e has been compared (Table1). The structures of isolated products were fully confirmed by elemental analysis, IR and 1H-NMR spectroscopy. |
 |
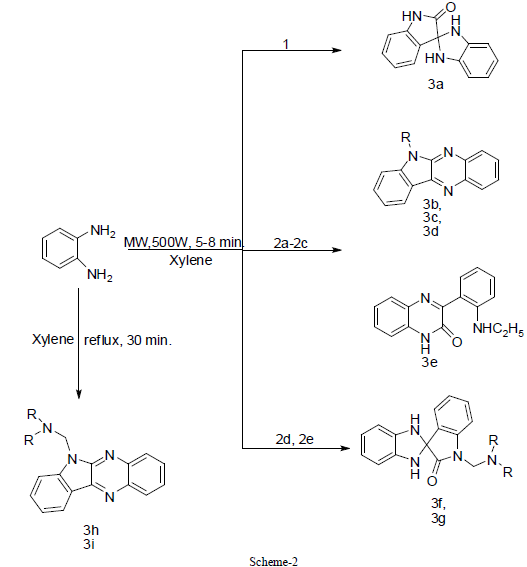
| Reaction of isatin and N- substituted isatins with equimolar amount of o- phenylenediamine in xylene under both conventional method and microwave irradiation leads to spiro-indole and quinoxaline derivatives. The microwave method was found to be better than conventional method in terms of reaction time, yield and relatively simple method to perform parallel synthesis. Thus this methodology becomes an efficient strategy for the rapid synthesis of spiroindole and quinoxaline derivatives (scheme 2). |
 |
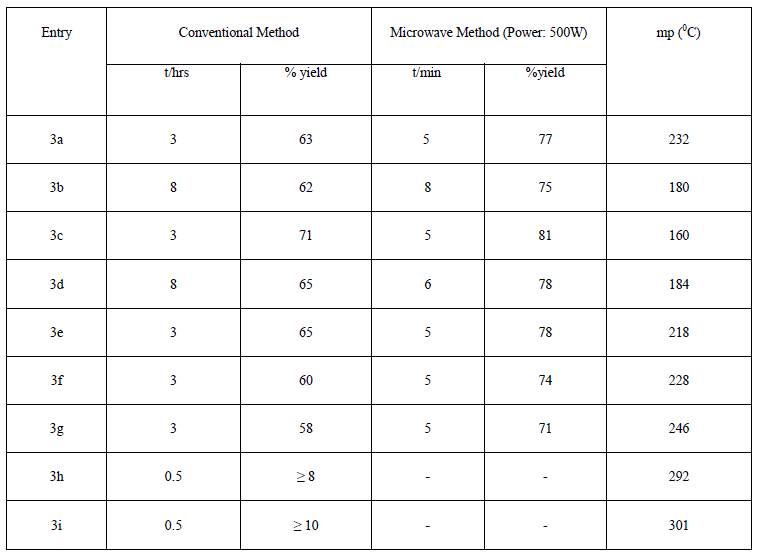
| The list of synthesized compounds and the comparative yield statement is shown in table 2. |
 |
| On the basis of previous work, it was concluded that cyclization of isatin and N-substituted isatins with o-phenylenediamine is solvent dependent reaction [24]-[26]. The synthesis of quinoxaline [27] derivative has been described by the reaction of N-substituted isatin with o-phenylenediamine using xylene as a refluxing solvent. This encourage us to use xylene as a refluxing solvent for the condensation reaction between isatin, N-substituted isatins and o- phenylenediamine. In order to optimize the reaction condition, we have carried out reactions at different time intervals. Interesting results were obtained by stopping the reaction of 2d and 2e in 0.5 hr. The quinoxaline derivatives 3h and 3i were obtained after workup, but the yield was very low (approx. 10%), while spiro-indole derivatives 3f and 3g were obtained by stopping the reaction after 3 hr as sole product in good yield. No further change of product was observed with increasing reaction time from 3 to 18 hr, whereas under microwave irradiation instead spiro-indole derivatives 3f and 3g were obtained as a sole product and there is no trace of quinoxaline derivative. On the other hand, the spiro-indole 3a was obtained selectively from 1. Now, the quinoxaline derivatives 3c and 3d were obtained from 2b and 2c as a sole product in good yield by both conventional as well as microwave irradiation method. In the case of Nethylisatin 2a, 3b and 3e were obtained in 8 hr (MW 8 min) and 3 hr (MW 5 min) respectively (Scheme2). The presence of peak around 1703cm-1 for carbonyl group in IR-spectrum supports the presence of spiro-indole compounds 3a, 3f and 3g, whereas absence of carbonyl group peak and presence of peak around 1660cm-1 in IR spectrum confirms the quinoxaline derivatives 3b-3d, 3h & 3i. The absence of NH2 peak in IR spectrum is in agreement of the proposed derivatives [24] which is also in good agreement with those observed by Nume and co-workers for such compounds. |
| The structures of isolated products were fully confirmed by elemental analysis and spectroscopic methods (IR & 1HNMR). |
EXPERIMENTAL |
| A. General: All chemicals were procured from Aldrich, USA, and E. Merck, Germany and used as such. TLC was carried out on SiO2 gel (HF254, 200 mesh). The solvent system employed was ethyl acetate: chloroform (11: 9) and the spots were identified by placing the plate in Iodine chamber. IR spectra were recorded on a PerkinElmer FT/IR version 10.03.05 spectrometer. NMR spectra were run on a JEOL AL300 FTNMR spectrometer; chemical shifts are given in δ ppm, relative to TMS as internal standard. Elemental microanalysis was performed on Exeter Analytical Inc Model CE- 440 CHN Analyzer. Melting points were measured in open capillaries and are uncorrected. The microwave assisted reactions are carried out in a “MAS-II, Microwave Synthesis System” manufactured by Sineo microwave chemistry technology co. Ltd, having an output energy range of 0 to 1000 watts, based on temperature of reaction and individual sensor for temperature control with attachment of reflux condenser with both magnetic and mechanical stirring device (thus avoiding the risk of high pressure development) and synthesis on preparative scales. |
| B. General procedure for the synthesis of compounds (2a-2c) |
| Conventional Method |
| Isatin (0.001mol) and halohydrocarbon (0.0012 mol) were dissolved in DMF (20 ml), and anhydrous K2CO3 (0.003 mol) was added. The mixture was stirred for 10-14 hr under room temperature until the disappearance of isatin, the completion of the reaction was monitored by TLC. The solvent was removed in vacuo and the residue was separated by column chromatography (silica gel, petroleum ether/ethyl acetate = 20:1), giving N-alkyl substituted isatin compound. Microwave Irradiation Method Isatin (0.001 mol) and halohydrocarbon (0.0012 mol) were dissolved in DMF (20 ml), and anhydrous K2CO3 (0.003 mol) was added to it and the reaction mixture was irradiated in microwave oven at power (500W) for 9-12 min. The completion of the reaction was monitored by TLC. The solvent was removed in vacuo and the residue was separated by column chromatography (silica gel, petroleum ether/ethyl acetate = 20:1), giving N-alkyl substituted isatin compounds. |
| C. General procedure for the synthesis of compounds (2d, 2e) |
| Conventional Method |
| Secondary amine (0.004 mol) in 10 ml of ethanol was added to slurry containing appropriate isatin (0.004 mol) and 0.3 ml of formaldehyde solution (37% v/v) in 10 ml of ethanol. The reaction mixture was stirred for 6 hr at room temperature and the completion of the reaction was monitored by TLC. Reaction mixture was then refrigerated for 48 hr. The products were separated by suction filtration, vacuum dried and recrystallized from ethanol. Microwave Irradiation Method Secondary amine (0.004 mol) in 10 ml of ethanol was added to slurry containing appropriate isatin (0.004 mol) and 0.3 ml of formaldehyde solution (37% v/v) in 10 ml of ethanol. The reaction mixture was then irradiated in microwave oven at power (500W) for 8 min and the completion of the reaction was monitored by TLC. Reaction mixture was then refrigerated for 48 hr. The products were separated by suction filtration, vacuum dried and recrystallized from ethanol. |
| D. Characterization data for the synthesized compounds (2a-2e) |
| 1-Ethylindoline-2,3-dione(2a) [30]: Dark-red solid. IR (KBr): vmax: 3064, 2987, 1738, 1727, 1610, 1469, 1354, 1289, 1158, 1162, 759, 701 cm-1. 1H-NMR (CDCl3) δ: 7.65- 6-92 (m, 4H, Ar-H), 3.86 (q, 2H, J=7.2, CH2 ), 1.30 ( t, 3H, J=6.9, CH3). Anal. Calc. For (C10H9NO2): C, 68.60; H, 5.21; N, 7.99; O, 18.20 (%). Found: C, 68.58; H, 5.22; N, 8.01; O, 18.19 (%). Ethyl 2-(2,3-dioxoindolin-1-yl)acetate(2b) [31]: Yellow-brown solid. IR (KBr): vmax: 3054, 2986, 1746, 1615, 1470, 1347, 1341, 1277, 1213, 1176, 1096, 758, 715 cm-1. 1H-NMR (CDCl3,) δ: 7.70- 6.72 (m, 4H, Ar-H ), 4.40 (s, 2H, CH2 ), 4.18 (q, 2H, J=7.2, CH2), 1.24 (t, 3H, J=7.2, CH3 ). Anal. Calc. For (C12H11NO4): C, 61.82; H, 4.74; N, 6.00; O, 27.44 (%). Found: C, 61.80; H, 4.75; N, 6.02; O, 27.43 (%). 1-(4-Bromobutyl)indoline-2,3-dione(2c): Reddish- yellow solid. IR (KBr): vmax: 3087, 2928, 1733, 1605, 1467, 1360, 1341, 1189, 1162, 753, 735 cm-1. 1H NMR (CDCl3) δ: 7.72-7.21 (m, 4H, Ar-H), 4.19 (t, 2H, J=7.5, CH2), 3.6 (t, 2H, J=7.2, CH2), 1.97 (m, 2H, CH2), 1.63 (m, 2H, CH2).Anal. Calcd. For C12H12BrNO2 (282.13): C, 51.09; H, 4.29; Br, 28.32; N, 4.96; O, 11.34 (%). Found: C, 51.10; H, 4.28; Br, 28.30; N, 4.97; O, 11.35 (%). |
| 1-[(Diphenylamino)methyl]indoline-2,3-dione(2d) [32]: Light- red solid. IR ( KBr): vmax: 3030, 2850, 1728, 1616, 1460, 1402, 1331, 770, 735 cm-1. 1H NMR (CDCl3) δ: 7.82-6.73 (m, 14H, Ar-H), 4.98 (s, 2H, CH2). Anal. Calcd. For C21H16N2O2 (328.36 ): C, 76.81; H, 4.92; N, 8.53; O, 9.74 (%). Found: C, 76.80; H, 4.93; N, 8.51; O, 9.76 (%). 1-[(Diethylamino)methyl]indoline-2,3-dione(2e) [32]: Red solid. IR (KBr): vmax: 2963, 2873, 1732, 1610, 1471, 1352, 766, 755, cm-1. 1H NMR (CDCl3) δ: 7.8-7.3 (m, 4H, Ar-H), 4.41 (s, 2H, CH2), 2.68(q, 4H, J=7.0, CH2), 1.55 (t, 6H, J=6.9, CH3). Anal. Calcd. For C13H16N2O2 (232.28): C, 67.22; H, 6.94; N, 12.06; O, 13.78 (%). Found: C, 67.23; H, 6.92; N, 12.06; O, 13.79 (%). |
| E. General procedure for the synthesis of compounds (3a-3g) |
| Conventional Method |
| Equimolar quantity of N-substituted isatin [(2a-2e), (0.001 mol)] and o-phenylenediamine (0.001 mol) in xylene (30 ml) was refluxed for 3-8 hr.The completion of the reaction was monitored by TLC. The reaction mixture was refrigerated for few hours and then filtered, dried, and recrystallized in ethanol. Microwave Irradiation Method Equimolar quantity of N-substituted isatin (0.001 mol) and o-phenylenediamine (0.001 mol) in xylene (30 ml) was irradiated in microwave oven at power (500W) for 5-8 min and refrigerated for few hours and then filtered, dried, and recrystallized in ethanol. |
| F. General procedure for the synthesis of compounds (3h &3i) |
| Equimolar quantity of mannich bases of isatin [(2d, 2e), (0.001 mol)] and o-phenylenediamine (0.001 mol) in xylene (30 ml) was refluxed for 0.5 hr. The reaction mixture was then refrigerated for few hours and filtered, dried, and recrystallized in ethanol. |
| G. Characterization data for the synthesized compounds (3a-3i) |
| 1,3-Dihydrospiro[benzo[d]imidazole-2,3'-indolin]-2'-one(3a): Brown-yellow solid. IR (KBr): vmax: 3192, 3090, 3019, 1703, 1614, 1598, 1484, 1339, 718, 671 cm-1. 1H NMR (DMSO) δ: 12.02 (s, 1H, NH), 8.35- 6.53 (m, 8H, Ar-H ), 6.43 [ s, 1H (D2O Exchangeable), NH- Spiro system]. Anal. Calcd. For C14H11N3O (237.26), C, 70.87; H, 4.67; N, 17.71;O, 6.74. Found: C, 70.85; H, 4.68; N, 17.72; O, 6.74. 6-Ethyl-6H-indolo[2,3-b]quinoxaline(3b): Yellow solid. IR (KBr): vmax: 2962, 2853, 1660, 1611, 1567, 1482, 1448, 1427, 1378, 1331, 757, 738 cm-1. 1H NMR (CDCl3) δ: 8.49-7.35 (m, 8H, Ar-H), 4.56 (q, 2H, J=7.2, CH2), 1.52 (t, 3H, J=6.9, CH3). Anal. Calcd. For C16H13N3 (247.29),C, 77.71; H, 5.30; N, 16.99 (%). Found: C, 77.73; H, 5.29; N, 16.98 (%). Ethyl 2-(6H-indolo[2,3-b]quinoxalin-6-yl)acetate(3c): Yellow solid. IR (KBr): vmax: 2976. 2927,1734, 1663, 1606, 1565, 1474, 1375, 1203, 760, 743 cm-1. 1H NMR (CDCl3) δ: 8.50-7.34 (m, 8H, Ar-H), 5.24 (s, 2H, CH2), 4.24 (q, 2H, J=7.2 CH2), 1.28 (t, 3H, J=7.2 CH3). Anal. Calcd. For C18H15N3O2 (305.33). C,70.81; H, 4.95; N, 13.76; O, 10.48 (%). Found: C, 70.80; H, 4.96; N, 13.75; O, 10.49 (%). 6-(4-Bromobutyl)-6H-indolo[2,3-b]quinoxaline(3d): Yellow solid. IR (KBr): vmax: 3050, 2930, 1660, 1493, 1485, 1450, 1400, 1330, 1192, 1130, 751, 741 cm-1. 1H NMR (CDCl3) δ: 8.53-7.38(m, 8H, Ar-H), 4.42 (t, 2H, J=7.5, CH2), 3.38 (t, 2H, J=7.2, CH2), 2.02 (m, 2H, CH2), 1.82 (m, 2H, CH2). Anal. Calcd. For C18H16BrN3 (354.24). C, 61.03; H, 4.55; Br, 22.56; N, 11.86 (%). Found: C, 61.01; H, 4.57; Br, 22.57; N, 11.85 (%). 3-[2-(Ethylamino)phenyl]quinoxalin-2(1H)-one(3e): Red solid. IR (KBr): vmax: 3385, 3364, 3288, 3189, 3027, 2962, 2853, 1659, 1620, 1592, 1569, 1457, 1379, 1332, 749, 724 cm-1. 1H NMR (CDCl3) δ: 9.50 (s, 1H, NH ), 8.51 (s, 1H, NH ), 7.83-7.37 (m, 8H, Ar-H ), 4.36 (q, 2H, J=7.2 CH2 ), 1.58 (t, 3H, J=6.8, CH3 ) . Anal. Calcd . For C16H15N3O (265.31), C, 72.43; H, 5.70; N, 15.84; O, 6.03 (%). Found: C, 72.45; H, 5.69; N, 15.82; O, 6.04 (%). 1'-[(Diphenylamino)methyl]-1,3-dihydrospiro[benzo[d]imidazole-2,3'-indolin]-2'-one(3f): Brown solid. IR (KBr): vmax: 3090, 2980, 2850, 1703, 1614, 1567, 1483, 1350, 744, 752 cm-1. 1H NMR (DMSO) δ: 8.35- 6.56 (m, 18H, Ar-H), 6.45 [ s, (D2O Exchangeable), NH-Spirosystem], 5.21 (s,2H,CH2). Anal. Calcd. For C27H22N4O (418.49). C, 77.49; H, 5.30; N, 13.39; O, 3.82 (%). Found: C, 77.50; H, 5.30; N, 13.38; O, 3.82 (%). 1'-[(Diethylamino)methyl]-1,3-dihydrospiro[benzo[d]imidazole-2,3'-indolin]-2'-one(3g): Yellow solid. IR (KBr): vmax: 3384, 2850, 1703, 1613, 1598, 1483, 1322, 759, 743 cm-1. 1H NMR (DMSO) δ: 8.07-6.53 (m, 8H, Ar-H), 6.42 [s, (D2O Exchangeable), NH-Spirosystem], 4.72 (s, 2H, CH2), 2.89 (q, 4H, J=7.0, CH2), 1.63 (t, 6H, J=6.9 CH3). |
| Anal.Calcd. For C19H22N4O (322.40), C, 70.78; H, 6.88; N, 17.38; O, 4.96(%). Found: C, 70.75; H, 6.89; N, 17.41; O, 4.95 (%). N-[(6H-indolo[2,3-b]quinoxalin-6-yl)methyl]-N-phenylaniline(3h): Greenish brown solid IR(KBr): vmax: 3100, 2845, 1663, 1611, 1538, 1461, 1275, 860, 759, 735 cm-1. 1H NMR (CDCl3) δ: 8.27-6.91 (m, 18H, Ar-H), 5.59 (s, 2H, CH2). Anal. Calcd. For C27H20N4 (400.47): C, 80.93; H, 5.04; N, 14.02 (%). Found: C, 80.93; H, 5.03; N, 14.01(%). N-[(6H-indolo[2,3-b]quinoxalin-6-yl)methyl]-N-ethylethanamine(3i): Light green solid. IR(KBr): vmax: 3351, 2845, 1663, 1612, 1538, 1461, 1287, 860, 759, 747 cm-1. 1H NMR (CDCl3) δ: 8.14-7.75 (m, 8H, Ar-H), 4.62 (s, 2H, CH2), 2.74(q, 4H, J=7.0, CH2), 1.24 (t, 6H, J=6.9, CH3). Anal. Calcd. For C19H20N4 (304.39): C, 74.92; H, 6.68; N, 18.40 (%). Found: C, 74.91; H, 6.67; N, 18.42 (%). |
CONCLUSION |
| We have developed a simple and efficient MW assisted approach for the synthesis of quinoxaline derivatives and spiroindoles via condensation of isatin and N-substituted isatins with o-phenylenediamine in xylene. MW irradiation offers many advantages over conventional heating: it facilitating reaction workup, requires less solvent, decreases reaction times and increases yields. |
ACKNOWLEDGEMENT |
| The authors would like to acknowledge the financial support provided by BHU, Varanasi and UGC, New Delhi for JRF. |
References |
|