ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| V. Sridevi, M.V.V.Chandana Lakshmi, Satya vani yadla Associate Professor, Dept. of Chemical Engg., Andhra University, Vizag – 530003, India |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Adsorption is one of the most widely applied techniques for environmental remediation. Burning pulverized coal in thermal power plants (TPPs) generates large amounts of fly ash (FA) that must be disposed of or otherwise handled; in an environmentally sound manner. Fly ash could be used as low cost adsorbent material for waste water treatment. Experiments were designed to investigate the removal of lead ions from aqueous solution onto the fly ash have been carried out at room temperature under the batch mode. The effect of various parameters such as agitation time, adsorbent dosage and initial concentration on the extent of removal of lead has been determined. The percentage removal of Pb (II) ions was found to increase with decrease in initial concentration and increase with increase in contact time and dosage of adsorbent. The adsorption process was fast and equilibrium was achieved in about 60 min of contact. The maximum removal of lead was 90.37% obtained at the adsorbent dosage 1.5 g/L. At an optimum agitation time of 60 min, the percentage removal of lead from the aqueous solution is decreased from 91.72% to 83.52% by varying lead concentrations in the aqueous solution from 20mg/l to 140mg/l. Experimental data were analyzed by model equations such as Langmuir and Freundlich isotherms and it was found Freundlich isotherm model best fitted well the adsorption data.
Keywords |
| Adsorption, Adsorbent, Adsorption isotherms, Lead, Fly ash. |
I. INTRODUCTION |
| Environmental contamination by toxic metals is a serious problem worldwide due to their incremental accumulation in the food chain and continued persistence in the ecosystem. The heavy metals lead, mercury, copper, cadmium, zinc, nickel, chromium is among the most common pollutants found in industrial effluents. Even at low concentrations, these metals can be toxic to organisms, including humans. For instance, lead is a naturally occurring element and a metal which is extremely toxic and it has adverse effects as it accumulates in the body, especially for children and pregnant women. It can lead to behavior disorders, anemia, mental retardation and permanent nerve damage [10, 28]. Lead pollution primarily comes from manmade sources cars used gas with lead, lead smelters, production of storage batteries, metal processing plants and incinerators [13]. Food is the major source of lead intake that who are not occupationally exposed or have high concentration of lead in drinking water. |
| A characteristic of non-degradability of metals associated with the accumulation capacity in environmental compartments resulted in the development of a range of methods and materials capable of separating or removing species such as lead, copper, cadmium, nickel and other elements from natural waters and industrial effluents[6, 5, 31]. There are many conventional techniques to reduce environmental problems associated with industrial effluents. Some of these techniques are chemical precipitation, chemical reduction or oxidation, filtration, reverse osmosis, filtration, solvent extraction, membrane filtration and adsorption. Every technique has its own advantage and disadvantage. Now a day, adsorption technique remains widely preferred due to its simplicity of design and operation, cost effective, high efficiency and flexibility [14]. Fly ash is an adsorbent that is inexpensive and readily available. The application of fly ash for the treatment of industrial effluents is gaining attention as a cost effective and simple. As an adsorbent, this is a surface phenomenon that depends on the higher specific surface area, narrow particle size distribution and the porosity of adsorbent. |
| The present study deals with the utilization of low cost adsorbents such as fly ash for adsorption of lead metal ions from aqueous solution. The main aim of this research was to determine the effects of different parameters such as agitation time, adsorbent dosage and initial concentration of the solution which were effective at the removal of Pb (II) ions from aqueous solutions [1,2] |
II. METHODS AND MATERIALS |
| 2.1 Preparation of adsorbent |
| The fly ash used in this experiment was collected as a solid waste material of the power plant from “National Thermal power Corporation”, Vizag, Andhra Pradesh, India. The fly ash obtained from burning of coal was dried and sieved into different fractions using Rotap sieve shaker. The fly ash was preserved in glass bottles for use as an adsorbent. |
| 2.2 Preparation of adsorbate |
| Stock solution of lead (1000 mg/L) was prepared by dissolving 1.6gm of 99% Pb(NO3)2 in 1L of double distilled water. The concentration range of lead prepared from stock solution varied between 10 to 100 mg/L. The pH of solution was adjusted with 0.1 mol l−1 NaOH and HCl. All the chemicals used were of analytical reagent grade[9]. |
| 2.3 Analysis |
| The concentration of lead in the solutions before and after equilibrium were determined by atomic absorption spectrophotometer (perckin elmer aa 110 model). The pH of solution was measured with a Hanna pH meter using a combination glass electrode [15]. |
| 2.4 Adsorption Experiment |
| In this experimentation the effects of various parameters such as agitation time, adsorbent dosage and initial concentration on adsorption were studied by fly ash. The pH of solution was maintained at a desired value by adding 0.1 M NaOH or HCl. The experimental procedure for determining various parameters was discussed [21] |
| 2.5 Effect of agitation time and adsorbent dosage |
| 50 ml of aqueous solution was taken in a 250ml conical flask and add 0.5g of adsorbent having the size of 52μm. This sample was shaken in an orbital shaker at room temperature for 1 hr. similarly eight samples were prepared in a conical flask adding 0.5g of adsorbent and exposed to varying agitation times (5min, 10min, 20min, 30min, 40min, 50min, 60min, 120min, 180min, and 240min). The samples were filtered with Whatman filter papers and filtrate was analyzed in Atomic Adsorption Spectrophotometer (AAS) to obtain final concentrations of lead[12,18]. The same experimental procedure was repeated with other adsorbent dosages (1.0 g, 1.5 g). The percentage removal of lead nitrate was calculated as |
| % removal = (Co – Ci) x 100/Co -------- (1) |
| Graphs are plotted between the contact time and percentage removal of lead nitrate separately to identify the optimum agitation time. The effects of adsorbent dosage on adsorptions were also obtained at this optimum agitation time. |
| 2.6 Effect of the initial concentration of the lead in aqueous solution |
| 50ml of mixed solution containing 50mg/l of lead nitrate was taken in a 250ml conical flask and exposed to 1.5gm of 52μm adsorbent size. The flasks were kept in an orbital shaker for optimum contact time (1 hr) at room temperature (300°K). Then the sample was allowed to settle and filtered. The final lead concentration of the filtrate was determined by using AAS [7,19]. The same procedure was repeated for other initial concentrations of lead in aqueous solution (20mg/l, 40mg/l, 60mg/l, 80mg/l, 100mg/l, 120mg/l and 140mg/l). |
III. RESULTS AND DISCUSSIONS |
| 3.1 Effect of agitation time |
| Adsorption of lead was measured at a varying contact time for initial concentration of 100 mg/L. from the Fig.1 and table -1, the plot reveals that the lead removal is higher at the beginning; this is probably due to a large surface area of the fly ash being available at the beginning for the adsorption of lead. As the surface adsorption sites become exhausted, the uptake rate is controlled by the rate at which the adsorbate is transported from the exterior to the interior sites of the adsorbent particles [32,35]. Most of the maximum percent lead removal was attained after about 60 min of shaking time. The increasing contact time increased the lead adsorption and it remained constant after equilibrium was reached in 240 min for initial concentrations. |
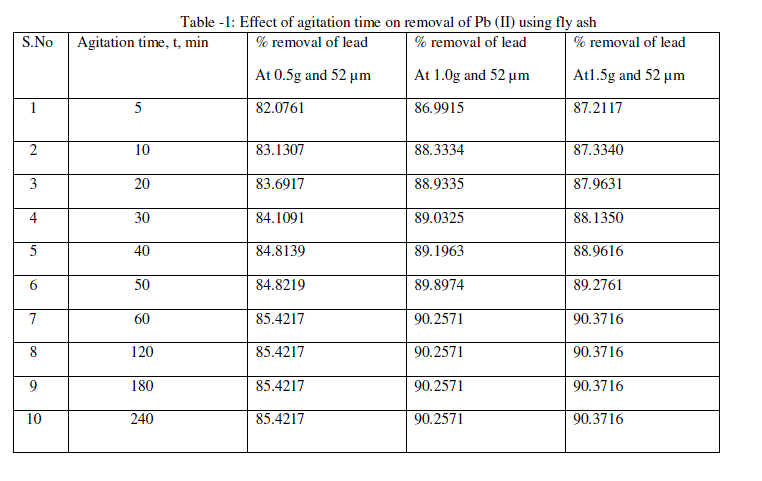 |
 |
| 3.2 Effect of adsorbent dosage |
| The effect of the adsorbent dosage was studied at room temperature by varying the adsorbent amounts from 0.5 to 1.5 g/L. For all these runs, the initial concentration of lead was fixed at 100 mg/L. Fig. 2 and table-2 shows that the adsorption of lead increases rapidly with an increase in the amount of fly ash due to greater availability of the surface area at higher concentrations of the adsorbent. A significant increase in uptake was observed when the dose was increased from 0.5 to 1.5 g/L. From the results, it is revealed that, within a certain range of initial metal concentration, the percentage of metal adsorption fly ash is determined by the adsorption capacity of the fly ash [22]. The maximum removal of lead was 90.37% obtained at the adsorbent dosage 1.5 g/L. |
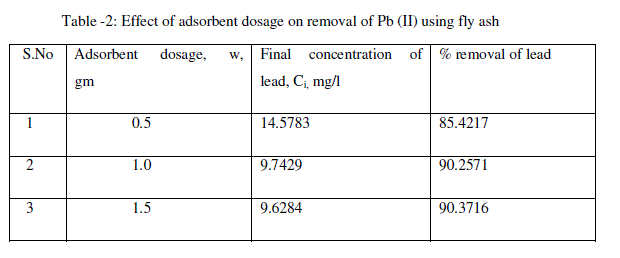 |
 |
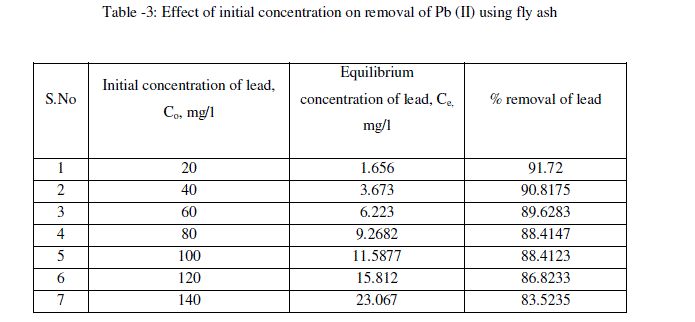
| 3.3Effect of the initial concentration of the lead in aqueous solution |
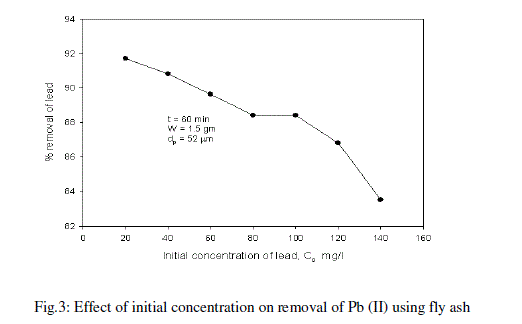
| The effect of initial concentration of lead in the aqueous solution on the percentage removal of lead at equilibrium is shown in Fig.3 and table -3 At an optimum agitation time of 60 min, the percentage removal of lead from the aqueous solution is decreased from 91.72% to 83.52% by varying lead concentrations in the aqueous solution from 20mg/l to 140mg/l. It is noticed from the Fig.3 that at lower concentrations of Pb (II), fly ash adsorbs more amounts of Pb (II). Lesser percentage of Pb (II) is removed at higher concentration in the aqueous solution [16]. Such behavior can be attributed to the increase in the amount of adsorbate to the unchanging number of available active sites on the adsorbent (since the amount of adsorbent is kept constant). |
 |
 |
| Adsorption isotherms |
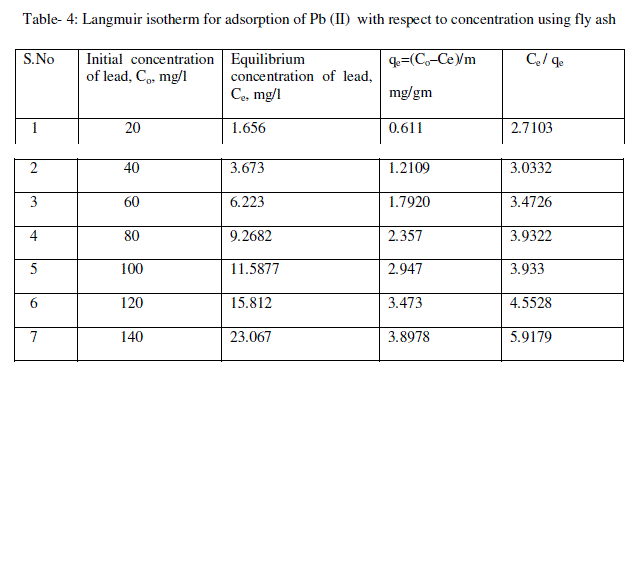
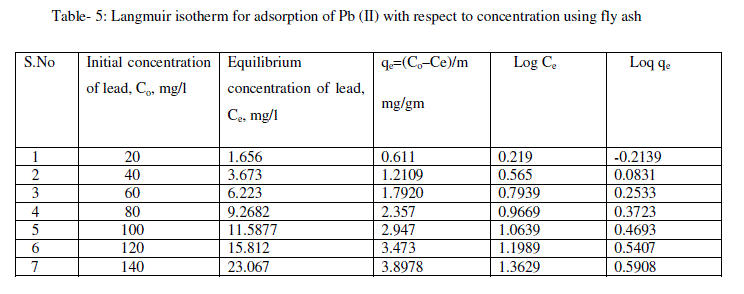
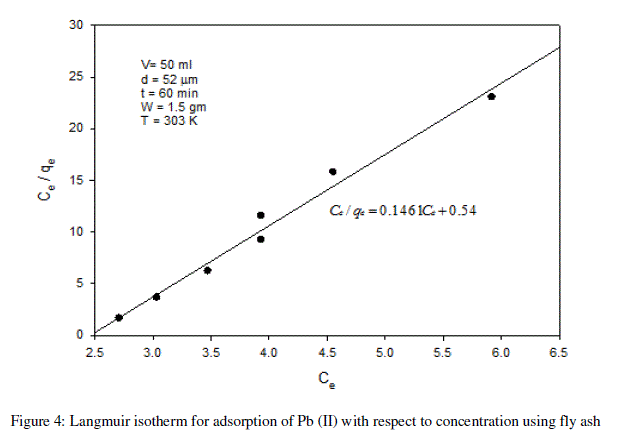
| 3.4.1 Langmuir isotherm |
| Since chemical forces fall off very rapidly with distance, it is probable that chemisorptions does not extend beyond a single layer of adsorbate on the surface of the solid. It can be anticipated as first pointed out by Langmuir that chemisorptions’ adsorbate layers may be only one molecule thick. Langmuir isotherm is the most widely used twoparameter equation. The relationship is a hyperbolic type form: |
| qe/qm = bCe/(1+bCe) --- (2) |
| Where Ce is the concentration of the adsorbate at equilibrium, qe is the amount adsorbed at equilibrium per unit mass of the adsorbent, qm is the maximum amount adsorbed and b is the coefficient related to affinity. Equation (2) can be rearranged as |
| (Ce/qe) = 1/bqm + Ce/qe --- (3) |
| From the plots drawn in fig 4 between (Ce/qe) and Ce, the slope (1/qm) and the intercept b/qm are calculated from the table 4 . |
 |
 |
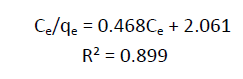 |
 |
| A further analysis of the Langmuir equation is made on the basis of separation factor, RL defined as |
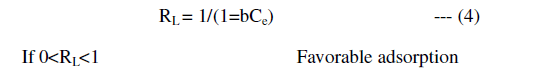 |
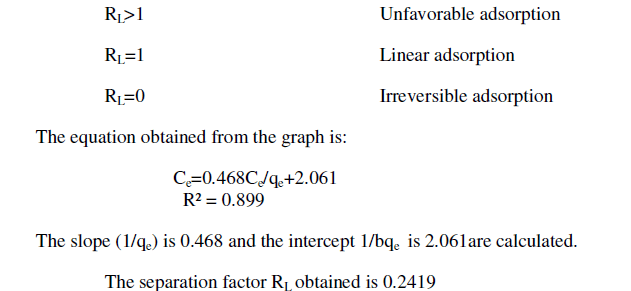 |
| In the present study, the Langmuir isotherm shows favorable adsorption indicating strong binding of Pb (II) to the surface of fly ash. |
| 3.4.2. Freundlich isotherm |
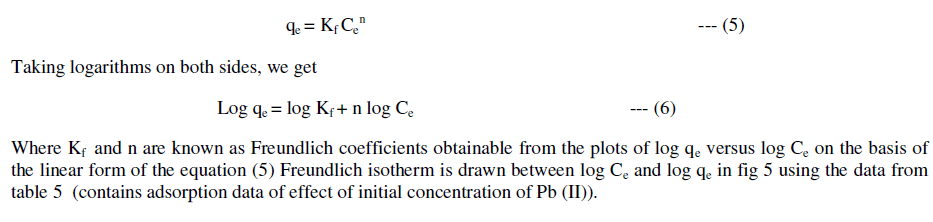
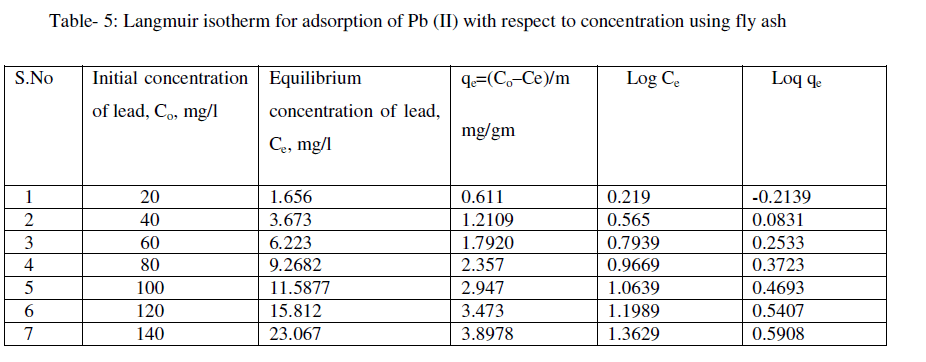
| The Freundlich isotherm is given by |
 |
 |
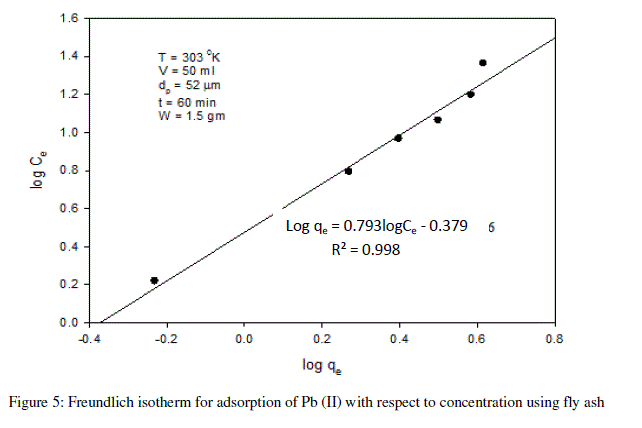 |
| The following equations are obtained from the graph: Log qe = 0.793logCe - 0.379 |
| R² = 0.998 |
| For the present data, the slope (n) of the above equation is less than one, fulfilling the Freundlich isotherm condition of 0 < n < 1. Hence, favorable adsorption. |
IV. CONCLUSION |
| Studies on kinetic parameters for adsorption of heavy metal Pb (II) ions from an aqueous solution using low cost and abundantly available adsorbent – fly ash. The optimum agitation time for the lead metal adsorption is 1hr for all concentrations. The percentage removal of lead from aqueous solution is augmented with increase with the adsorbent. Higher the concentration of lead in the aqueous solution decreases in the percentage removal of lead. In the range of variables studied, percentage adsorption is increased from 68.39% to 91.89%. The experimental data fulfills the Freundlich and Langmuir isotherm conditions indicating favorable adsorption of Pb (II) by fly ash and Freundlich is more favorable than Langmuir isotherm. |
References |
|