ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Ghanshyam G. Pandhare1, Nikhilesh Trivedi1, Rajesh Pathrabe2, S. D. Dawande3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The study on performance of low-cost adsorbent such as Neem leaves powder in the removal of Cadmium (II) and Lead (II) ion from aqueous solution is performed. In present study Neem Leaves Powder adsorbent prepared using chemical activation process and the surface area measured. The adsorbent material adopted was found to be an efficient media for removal of Cadmium (II) and Lead (II) ion with different parameter like Adsorbent dosages, Concentration and contact time etc. A comparative study has also been done on the adsorption capacity of Neem Leaves Powder. The column studies were conducted with different contact time and reduced concentration determines using Atomic Absorption Spectrophotometer (AAS). It was found that the metal uptake capacity (amount of removal) of Cadmium (II) and Lead (II) ion decreased but the adsorption capacity (percentage of removal) increased with the decrease in the concentration of Cadmium (II) and Lead (II) in the initial sample solution. Correlation coefficient and isotherm constant was also calculated from isotherm studies with different parameter
Keywords |
| Adsorption, Neem leaves Powder, Cadmium, Lead, Fixed column, Freundlich and Langmuir Isotherm |
INTRODUCTION |
| Adsorption is effectively in removing trace components from a liquid phase and may be used either to recover the components or simply to remove noxious substance from industrial waste. Any potential application of adsorption has to be considered along with alternatives such as distillation, absorption and liquid extraction. Each separation process exploits a difference between properties of substance to separate. In distillation the property applied is volatility, in absorption it is solubility, in extraction it is distribution coefficient. Separation by adsorption depends on one component being more readily adsorbed than other. The selection of a suitable process depends upon the ease with which the separated components are removed. |
| Adsorption is present in many natural physical, biological, and chemical systems, and is widely used in industrial applications such as activated charcoal, capturing and using waste heat to provide cold water for air conditioning and other process requirements (adsorption chillers), synthetic resins, increase storage capacity of carbidederived carbons for carbon, and water purification. |
| Ever increasing industrial activity is the main culprit behind most environmental pollution problems and ecosystem damage, coming from the accumulation of pollutants such as toxic metals chromium, copper, lead, cadmium, zinc, nickel, etc. [10]. Contamination of soils, groundwater, sediments, surface water and air with hazardous and toxic chemicals poses significant problems for both human health and the environment [11]. Heavy metals are considered to be particularly dangerous pollutants. |
| copper consumption in high doses brings about serious toxicological concerns since it can be deposited in the brain, skin, liver, kidney damage and anemia. Lead (Pb) has been cited as one of the three most toxic heavy metals that have latent long-term negative impacts on health, causing anemia, encephalopathy, hepatitis and nephritic syndrome [17]. Cadmium also exposes human health to severe risks, as it can provoke cancer, kidney damage, mucous membrane destruction, vomiting, bone damage as well as affect the production of progesterone and testosterone [15]. Moreover, the presence of nickel exceeding its critical level might bring about serious lung and kidney problems. |
| Cadmium can mainly be found in the earth's crust. Cadmium also consists in the industries as an inevitable byproduct of zinc an. After being applied it enters the environment mainly through the ground, because it is found in manures and pesticides. Naturally a very large amount of cadmium is released into the environment, Lead occurs naturally in the environment. However, most lead concentrations that are found in the environment are a result of human activities. Due to the application of lead in gasoline an unnatural lead-cycle has consisted. In car engines lead is burned. These lead salts enter the environment through the exhausts of cars. The larger particles will drop to the ground immediately and pollute soils or surface waters, the smaller particles will travel long distances through air and remain in the atmosphere. Lead is a soft metal that has known many applications over the years. It has been used widely since 5000 BC for application in metal products, cables and pipelines, but also in paints and pesticides. Lead is one out of four metals that have the most damaging effects on human health. |
MATERIALS AND METHOD |
| The Neem tree (Azadirachta indica A. Juss.) has been known as the wonder tree for centuries in the Indian subcontinent. It has become important in the global context today because it Offers answers to the major concerns facing mankind. The history of the Neem tree is inextricably linked to the history of the Indian way of life. Although the antiquity of Neem is shrouded in the mists of time, this evergreen robust looking tree has long been cherished as a symbol of health in the country of its origin. It has, for a very long time, been a friend and protector of the Indian village. It contains recommendations for specific trees to be planted in the vicinity of one’s house. Neem was highly recommended. The medical properties of Neem have been known to Indians since time immemorial. |
| A. Objective of the Present Work: |
| The objective of the present work is conversion of agricultural waste material like Neem leaves into adsorbent, characterization and study of utility in removal of two metals namely Cd and Pb from aqueous solution. The present Work is divided into following parts: |
| Synthesis of adsorbent by using chemical activation thermal technique |
| Characterization of adsorbent |
| Adsorption study for removal of Cd and Pb from aqueous solution |
| B. Characterization of the synthesized adsorbent obtained from Neem leaves powder by BET method: The surface area of carbon obtained from Neem leaves powder is determined by Brunauer, Emmett and Teller (BET) N2 sorption procedure with liquid N2 at 217.15oC. The specific surface area is found to be 421 m2/g. |
| C. Batch Adsorption Experiments: |
| Batch experiments are performed with 100ml of a sample solution and a desired adsorbent dose of 1 to 5 gm and parameter like contact time, concentration of solution. The mixture is transferred into a 250 ml conical flask and stirred for 48 hour, filtered and analyzed for its absorbance and concentration. All experiments are performed at room temperature conditions. Absorbance and concentration determine by Atomic absorption spectrophotometer. |
| D. Preparation of Absorbent |
| Initially Neem leaves were washed repeatedly by using distilled water to remove moisture and soluble impurities. Then neem leaves kept in dryer at 900C, for2-3 hrs till leaves turn pale yellow. Then crushed and screen by 10-15um mesh size. |
| Neem leaves powder washed to remove moisture and free acid and kept in dryer 20-25 minute. After drying powder was mixed with H3PO4 in silica crucible and kept in furnace at 2600c for 15-20 minute .The heating period depend on atmospheric temperature then solution was cooled & repeatedly washed using hot water to remove free acid and moisture, total 7 washing taken and kept it in dryer for 20-25 minute the prepared black colored adsorbent kept in bottle for further use |
| About 20 gm of sample and 10ml of Ortho-H3PO4 acid taken in silica crucible and kept in furnace. The furnace is initially at normal room temperature then furnace set at 2600C. Heating was carried out for 20 minute. Then sample was removed and cool. After cooling the sample was repeatedly washed for 7 times using hot water to remove free acid and moisture .Then sample kept in dryer for 20-25 minute and the activated black color adsorbent stored in bottle. |
| E. Preparation of Cadmium chloride solution |
| In a clean beaker 100 ml distilled water is heated, in this boiling water 0.6131 gm of Cadmium chloride added with constant stirring .Then solution shake well and cooled, then solution make 1000ppm and take 10ml of this to make 100ppm solution after this the various concentration of solution prepared for experimental procedure. |
| F. Preparation of Lead nitrite solution |
| In a clean beaker 100 ml distilled water taken add 0.3213 gm of Lead Nitrite with constant stirring .Then solution shake well, then solution make 1000ppm and take 10ml of this to make 100ppm solution after this the various concentration of solution prepared for experimental procedure. |
RESULTS AND DISCUSSION |
| Observation Table for CdCl2 Solution |
| Effect of Concentration: |
| Effect of initial metal ion concentration (C0) on the amount of metal adsorbed (q) at pH 6.5 is presented in table no. 1 The maximum quantities of q were achieved by chemically activated Neem leaves powder adsorbent. The q values increased with increase in initial ion concentration (C0). This increase is probably due to a high driving force for mass transfer. When the concentration of metal ions in solution was higher, the adsorption capacity increased. Absorbance and concentration determine by Atomic absorption spectrophotometer |
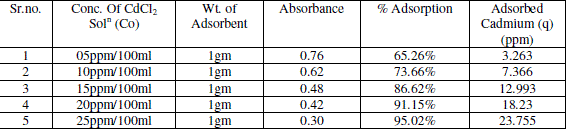 |
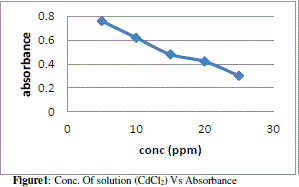
| The figure 1 shows the reduced absorbance of CdCl2 from different concentration of solution, It can be concluded that the concentration of solution reduced using 1gm of adsorbent and the adsorption of Cd increases at desired parameter. the % Adsorption for CdCl2 using 100ml solution of various concentrations with 1gm of adsorbent. And figure 2 indicates the % Adsorption with various amount of concentration. |
 |
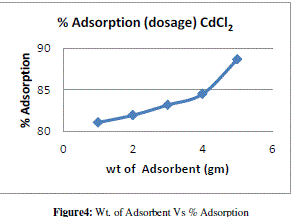
| Effects of Adsorbent Dosages |
| Adsorbent dosage is one of the important parameter of adsorption. The effect of adsorption dosage was determined at initial metal ions concentration of 10ppm/100ml and at pH 6.5. The results summarized in Table no. 2 indicate that, with the increase in dosage of adsorbent, the amount of metal ion concentration per unit mass of the adsorbent decreased but the percentage of adsorption increased. This effect can be due to some of adsorption sites remaining unsaturated during the adsorption reaction. |
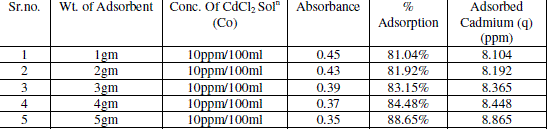 |
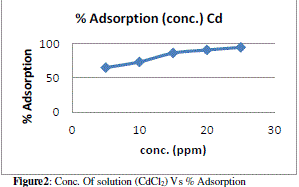
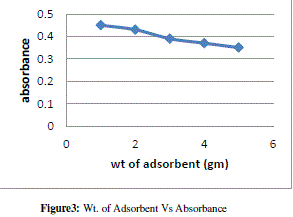
| The figure 3 shows the reduced absorbance of CdCl2 from different wt. of adsorbent, It can be concluded that the different wt. of adsorbent reduced the concentration of solution and the adsorption of Cd increases at desired parameter. the % Adsorption for CdCl2 solution using various weight of adsorbent and 10ppm/100ml CdCl2 solution figure 4 indicates the % Adsorption with various weight of adsorbent. |
 |
| Effect of Contact Time: |
| Contact time also one of the important parameter of adsorption. The effect of contact time was determined at initial metal ions concentration of 10ppm/100ml and at pH 6.5. The results summarized in Table 3 indicate that, with the increase in contact time, the amount of metal ion concentration per unit mass of the adsorbent decreased but the percentage of adsorption increased. This effect can be due to some of adsorption sites remaining unsaturated during the adsorption reaction. |
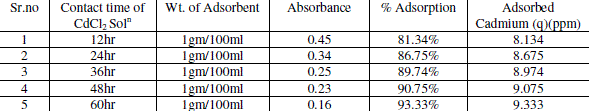 |
| The figure 5 shows the reduced absorbance of CdCl2 from different contact time, It can be concluded that the different contact time reduced the concentration of solution and the adsorption of Cd increases at desired parameter. Following fig. 6 shows the % Adsorption for CdCl2 solution using various contact time. |
 |
| Observation Table for Lead Nitrite Solution |
| Effect of Concentration |
| Effect of initial metal ion concentration (C0) on the amount of metal adsorbed (q) at pH 7 is presented in table no. 4 The maximum quantities of q were achieved by chemically activated Neem leaves powder adsorbent. The q values increased with increase in initial ion concentration (C0). This increase is probably due to a high driving force for mass transfer. When the concentration of metal ions in solution was higher, the adsorption capacity increased. Absorbance and concentration determine by Atomic absorption spectrophotometer |
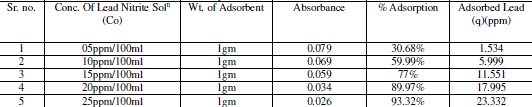 |
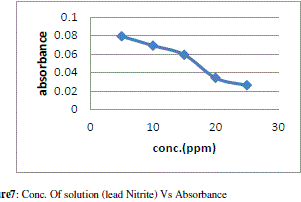
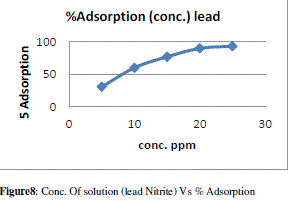
| The figure 7 shows the reduced absorbance of lead nitrite from different concentration of solution, It can be concluded that the concentration of solution reduced using 1gm of adsorbent and the adsorption of lead increases at desired parameter Following fig 8 shows the % Adsorption for 100ml lead nitrite solution with 1gm of adsorbent |
 |
| Effect of Adsorbent Dosages |
| Adsorbent dosage is one of the important parameter of adsorption. The effect of adsorption dosage was determined at initial metal ions concentration of 10 ppm/100ml and at pH 7. The results summarized in Table no 5 indicate that, with the increase in dosage of adsorbent, the amount of metal ions per unit mass of the adsorbent decreased but the percentage of adsorption increased. This effect can be due to some of adsorption sites remaining unsaturated during the adsorption reaction. |
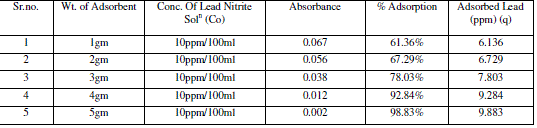 |
| The figure9 shows the reduced absorbance of lead nitrite from different wt. of adsorbent. It can be concluded that the different wt. of adsorbent reduced the concentration of solution and the adsorption of lead increases at desired parameter. the % Adsorption for lead nitrite solution using various weight of adsorbent and 10ppm/100ml solution of lead nitrite fig.10 shows % Adsorption using various amount of adsorbent dosages. |
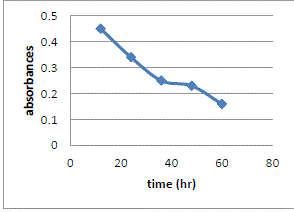 |
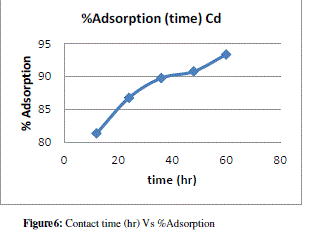
| Effect of Contact Time |
| Contact time also one of the important parameter of adsorption. The effect of contact time was determined at initial metal ions concentration of 10ppm/100ml and at pH 7. The results summarized in Table no. 6 indicate that, with the increase in contact time, the amount of metal ion concentration per unit mass of the adsorbent decreased but the percentage of adsorption increased. This effect can be due to some of adsorption sites remaining unsaturated during the adsorption reaction. |
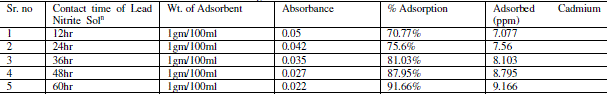 |
| The figure11 shows the reduced absorbance of lead nitrite from different contact time, It can be concluded that the different contact time reduced the concentration of solution and the adsorption of lead increases at desired parameter. the % Adsorption for lead nitrite solution using various weight of adsorbent and figure12 shows the % Adsorption using various contact time. |
 |
| Adsorption isotherm |
| Freundlich isotherm |
| The Freundlich isotherm model is the well-known earliest relationship describing the adsorption process. This model applies to adsorption on heterogeneous surfaces with the interaction between adsorbed molecules, and the application of the Freundlich equation also suggests that sorption energy exponentially decreases on completion of the sorption of an adsorbent. This isotherm is an empirical equation and can be employed to describe heterogeneous systems and is expressed as follows in linear form: |
| Original form: q = Kf C 1/n |
| Linearised form: log q = log Kf + (1/n) log C |
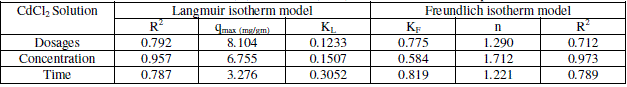
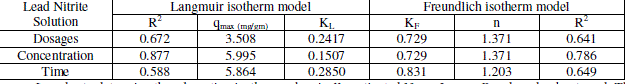
| Where Kf and 1/n is the Freundlich constant related to the bonding energy, 1/n is the heterogeneity factor and n is a measure of the deviation from linearity of adsorption. Freundlich equilibrium constants were determined from the plot of log q versus log C, on the basis of the linear of Freundlich equation. The n value indicates the degree of nonlinearity between solution concentration and adsorption as follows: if n = 1, then adsorption is linear; if n < 1, then adsorption is a chemical process; if n > 1, then adsorption is a physical process. The n value in Freundlich equation was found since n lies between 1 and 10, this indicates the physical biosorption of cadmium (II) and lead (II) |
| Langmuir isotherm |
| The Langmuir isotherm assumes monolayer adsorption on a uniform surface with a finite number of adsorption sites. Once a site is filled, no further sorption can take place at that site. As such, the surface will eventually reach a saturation point where the maximum adsorption of the surface will be achieved. The linear form of the Langmuir isotherm model is described as, |
| Original form: Q = (qm. KL. C)/ (1+ KL.C) |
| Linearised form: C/q = 1 / (KL.qm) + (C/qm) |
| Where KL is the Langmuir constant related to the energy of adsorption and qmax is the maximum adsorption capacity (mg/gm). Values of Langmuir parameters qmax and KL was calculated from the slope and intercept of the linear plot of 1/qe versus 1/C as shown in Figures. Values of qmax, KL, and regression coefficient R2 are listed. These values for Neem Leaves Adsorbent indicate that Langmuir theory describes the adsorption phenomena as favorable. The essential characteristics of the Langmuir isotherm parameters can be used to predict the affinity between the adsorbate and adsorbent using separation factor. |
| This equation straight line plot of log q Vs log C should be straight line with slope 1/n and intercept log K however it is actually found that the plot were straight line at low concentration, while at higher concentration they showed a straight curvature especially at low this indicate that Freundlich equation approximate and does not apply to adsorption of gases by solids at high conc. |
 |
 |
 |
 |
| In order to determine the adsorption isotherm, chemically activated Neem Leaves Powder adsorbent used. The experiments were performing at various parameters like concentration (ppm), weight of adsorbent (gm), contact time (hr). The effect of final concentration (C) on the Cadmium and lead adsorbed per specific mass of adsorbent q (mg/gm) is presented in figure. The shape of adsorption isotherm indicates that adsorption of Cadmium from CdCl2 solution and Lead from Lead Nitrite on Neem leaves powder adsorbent |
CONCLUSION |
| Batch Adsorption studies were performed for removal of Cadmium (II) and Lead (II) ion from stock solution using different concentration, adsorbent dosages, and contact time. The study indicated the suitability of the adsorbent for removal of Cadmium (II) and Lead (II) ion from aqueous solutions. The adsorbent may be viewed as a useful material while considering the economic aspects of wastewater treatment. The obtained results may be summarized as follows: |
| The metal uptake capacity decreases with decrease in the initial concentration of Cadmium (II) and Lead (II) solutions. However, the percentage of removal or the adsorption capacity is found to increase with lower initial values of different parameter. |
| The observed order of metal uptake capacity as well as adsorption capacity of Cadmium (II) and Lead (II) ion with different concentration, adsorbent dosages, and contact time parameter are 95.03%, 88.63% and 93.33% respectively and 93.32%, 98.83%, and 91.66% respectively. These experimental studies on the specified adsorbent would be quite useful in developing an appropriate technology for the removal of Cadmium (II) and Lead (II) ions from contaminated industrial effluents. |
| Future scope of work: |
| 1. Similar continuous column studies can be performed for locally available Neem leaves powder adsorbent to compare the metal uptake capacity and adsorption capacities. |
| 2. The metal uptake capacity and adsorption capacity can also be studied at different concentration, adsorbent dosages, pH and contact time. The column bed and by also varying the bed height. |
References |
|