ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
S. Saxena1*, Anuj Kumar Garg2, Ashu Rani3, A. K. Gupta2*
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Leaching kinetics of fluoride by loading AlF3 on undisturbed vertical saline soil columns has been investigated and compared using various kinetic models. Saline soils from Sambhar region of Rajasthan was selected to study leaching mechanism and effects of various parameters on leaching mechanism under atmospheric pressure. Linear relationship is established between the concentrations of leachable fluoride [F-]i and rate of leaching (LRobs). [F- ]i and LRobs are found to decrease with increase in Na+ and Ca+2 levels of extractant, while an increase has been observed with increase in temperature and OH- ions. Maximum [F-]i are resulted with addition of NH4OH in percolating water and minimum with addition of KOH. Total leachable F- was found to be unaffected by incubation time. First Order model are found to be best fit for representing fluoride leaching in the present experimental conditions.
Keywords |
| Leaching Kinetics, First Order Kinetic Model, Fluoride, Pore Volume, Saturated Flow, Saline Soil, Ion Exchange. |
INTRODUCTION |
| Fluoride is regarded as one of the most important environmental micro pollutant responsible for soil and groundwater pollution causing dental and skeletal fulorosis [1,2,3]. The risk for human health and the environment can largely be determined by the concentration of fluoride that occurs in groundwater and the rate by which fluoride migrates to groundwater as both these processes can strongly be influenced by the interaction of dissolved fluoride with the soil solid phase via adsorption and desorption [4] and cation-anion exchange. Thus it is important to study its migration and leaching. The study become more important in saline soils, as above pH 7.0, most of the inorganic fluoride salts are either complexed with Fe and Al show maximum solubility or remain in soluble ionic form in soil water [5]. Aluminium a dominant metal in earths’ atmosphere 7.5% when present naturally in large water bodies does not affect the water quality much but it reaches in drinking water predominantly through aluminium sulphate which is used in the coagulation process during water treatment. F- present in soil is bound in complexes and is usually transported through the water cycle dominantly complexed with aluminium. The maximum adsorption of fluoride is reported to occur at pH 5.5 [6]. In soils, with pH below 6, most of the fluoride remains complexed with either Al or Fe (e.g. AlF2+, AlF2 +, AlF3 0, AlF4 -, FeF2+, FeF2 +, FeF3 0). At pH 6, dissolved fluoride species in presence of Al are mainly AlF2+ (60.40%) whereas AlF2+, AlF3 0, AlF4 - remain dissolved upto 12.8– 0.08 % only. The free F- ion dissolution is approximately only 20% at pH 6. The dissolution of different aluminium fluoride decreases with increase in pH and at pH 8 and above, only free ionic fluoride, F-, remains 100 % dissolved [7]. Increasing amount of F- and Al+3 in medicine, industry and agriculture, drinks and other food products have made us concerned about its ill effect. AlFx influences the proper function of entire human body by affecting pituitary-thyroid gland system which has major role in regulating growth development and metabolism of many tissues etc [8]. Chronic exposure of humans to AlF3 begins in the foetus only. It may affect all pathological hallmark of the diseases by playing the role of a devil in various vital processes like neurotransmission, amyloid generation, transport of ions, energy metabolism, calcium homeostasis etc [9,10]. Thus by looking deep into the toxic effects of AlFx in plants and humans, the leaching of fluoride in fluoride endemic soil with pH 8.2 has been investigated by further adding AlF3 on to it in soluble form. In the present study, leaching kinetics of F- has been investigated in detail on AlF3 added columns. The effects of change in physico-chemical characteristics of percolating water such as Ca+2 hardness, Na levels, OH- ions and its associated cations, temperature etc have been studied on leaching rates. All the kinetic data are fitted on various kinetic models and a linear power form equation has been derived to show the relationship between added concentrations and initial leaching rates. |
II. MATERIAL & METHOD |
| Saline soil (pH= 8.2) has been collected from Sambhar region of Rajasthan, India and was dried in open air in sunlight. Dried soil was sieved for uniform particle size. The physico chemical properties of the soil used in columns are given in Table 1. The leaching kinetics of AlF3 has been studied by determining the fluoride concentrations in the leachate with time. Fluoride was estimated using Fluoride Ion Selective Electrode with TISAB [11]. Columns of soil were prepared surrounded by glass jacket of continuously flowing thermostated water. 60 g soil of pH 8.2 and of particle size (53>r) was gently packed at water filled porosity 0.315 cm3cm-3. The leachate’s pore volume was determined using equation (1) |
| Pv= q’ t /θ V (1) |
| where q' =Volume of effluent collected per unit time i.e. flow rate cm3 h-1 t =Time that has elapsed since the slug was introduced θ =water filled porosity cm3cm-3 V =Total volume of soil column |
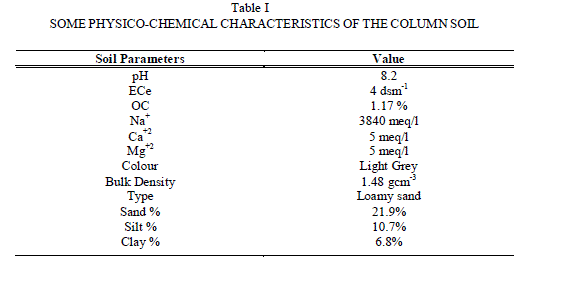 |
| The flow rate of extractant was found constant (2+0.5 ml/10 min). A fixed volume of aqueous salt solution (slug) with desired anion concentration was added at the top of the soil column in each experiment. Salt solution was allowed to get adsorbed uniformly in the column for 24 hrs, after which the columns was continuously leached with de-ionized water or with other extractant as per requirement of the study. The leaching was carried out till the soluble anions were completely removed. The total leachable concentration was taken equal to the total leachable concentration present initially during leaching (i.e. concentration t=0). During each kinetic run the concentration of ions were determined in leachate collected periodically at an interval of 2 min. The treatment of result obtained in leaching studies is based on calculations of initial leaching rates as well as on applications of various kinetic models for establishing the nature of leaching kinetics of water soluble fluoride salt. |
| The treatment of data is based on the calculation of the following parameters as defined below: |
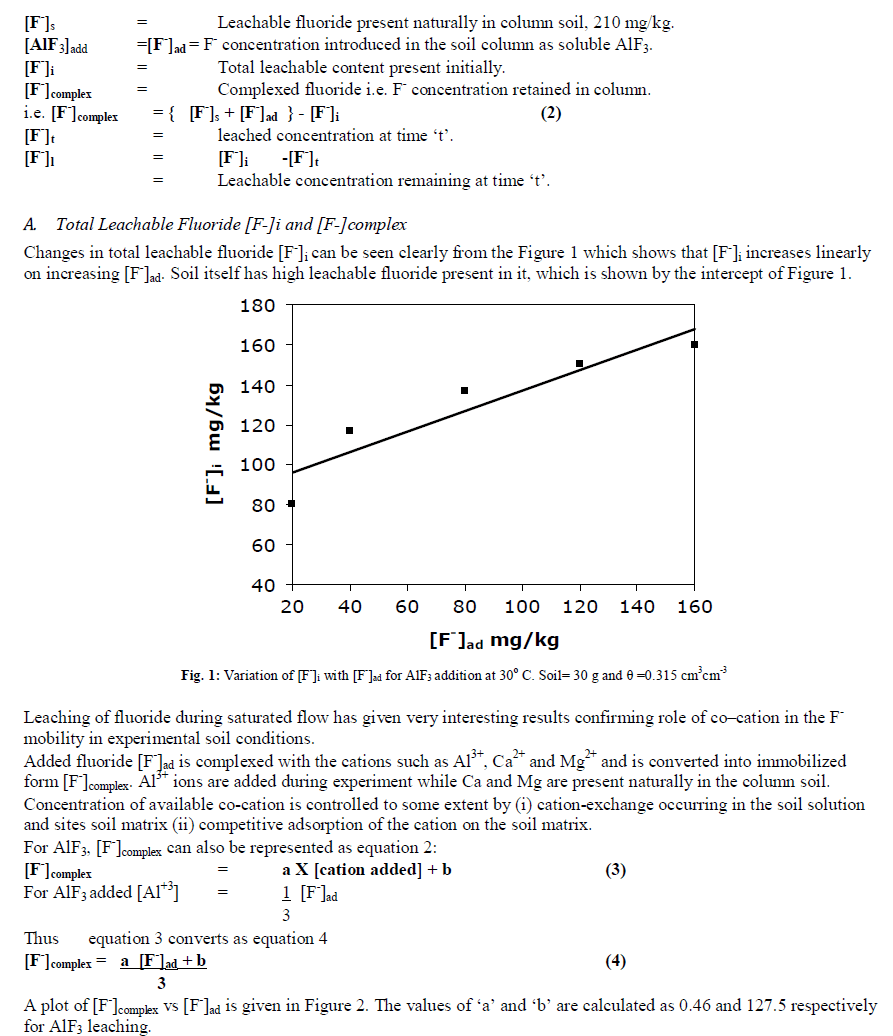 |
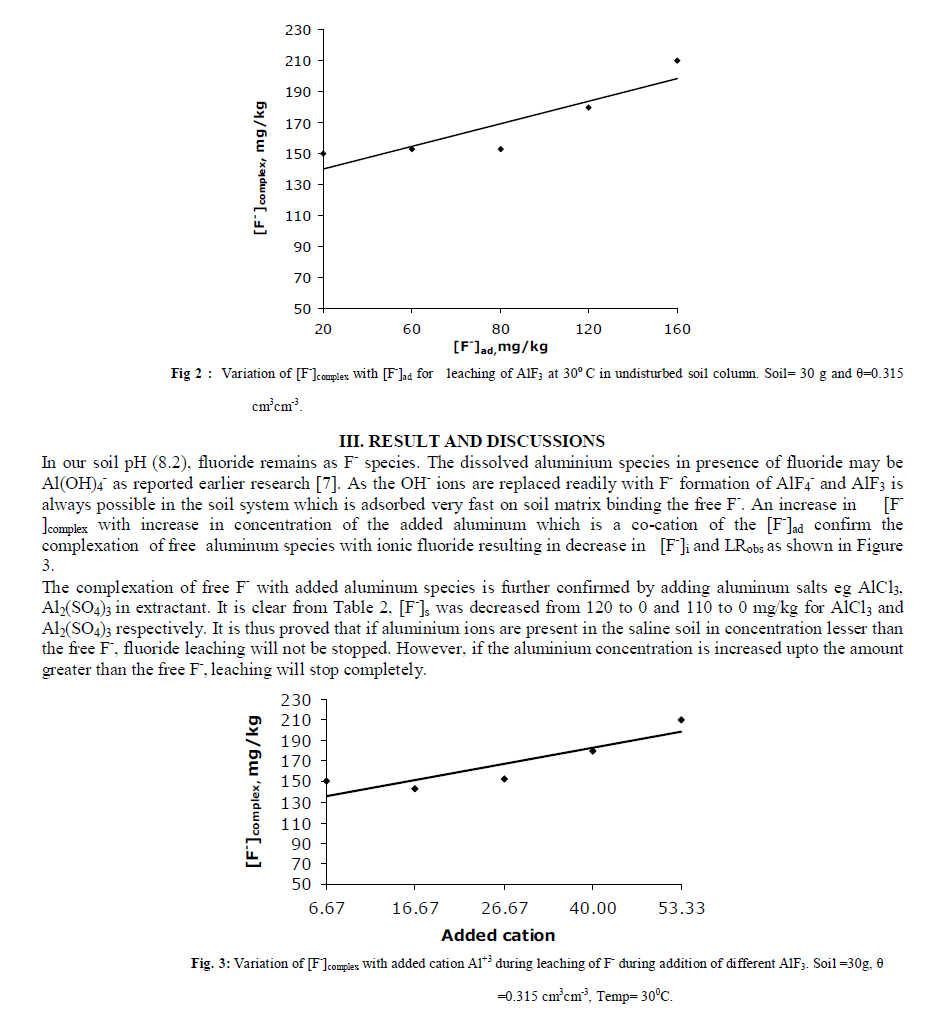 |
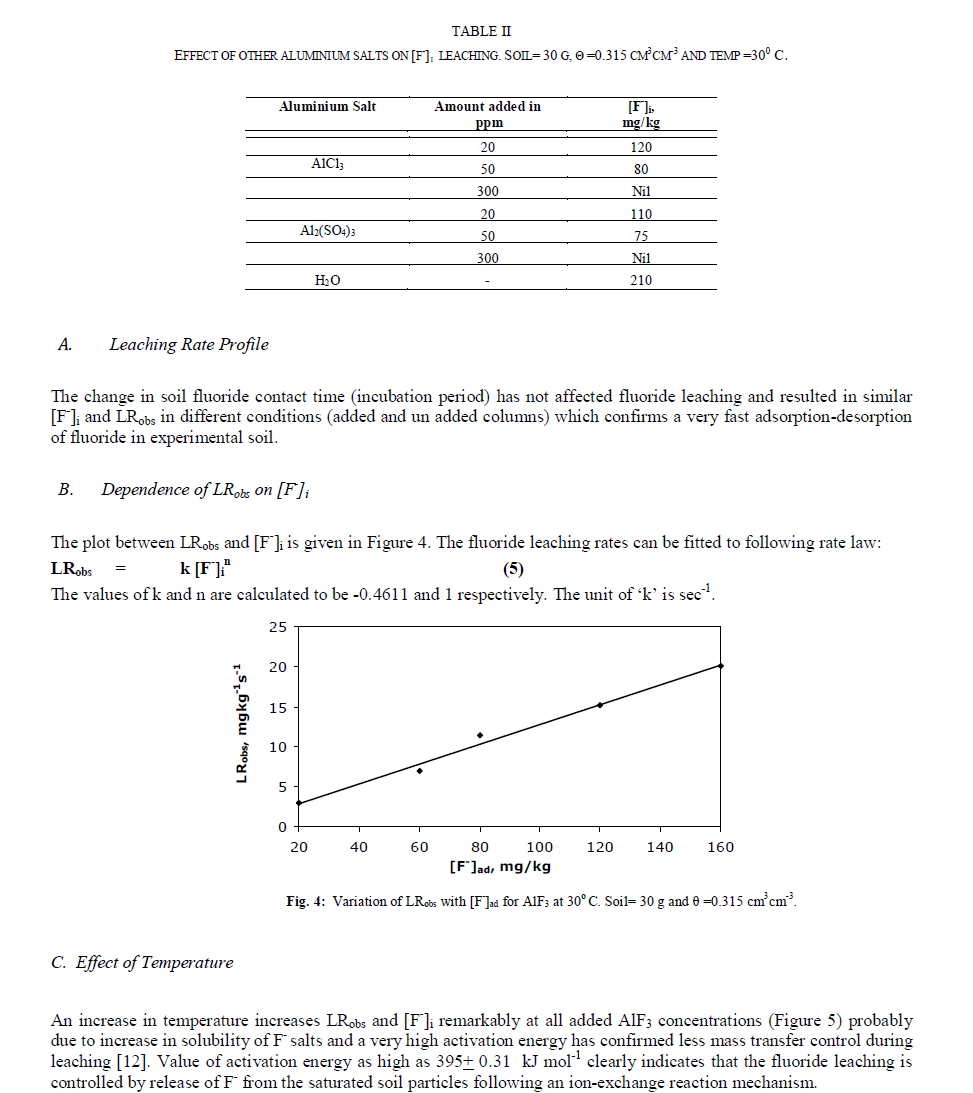 |
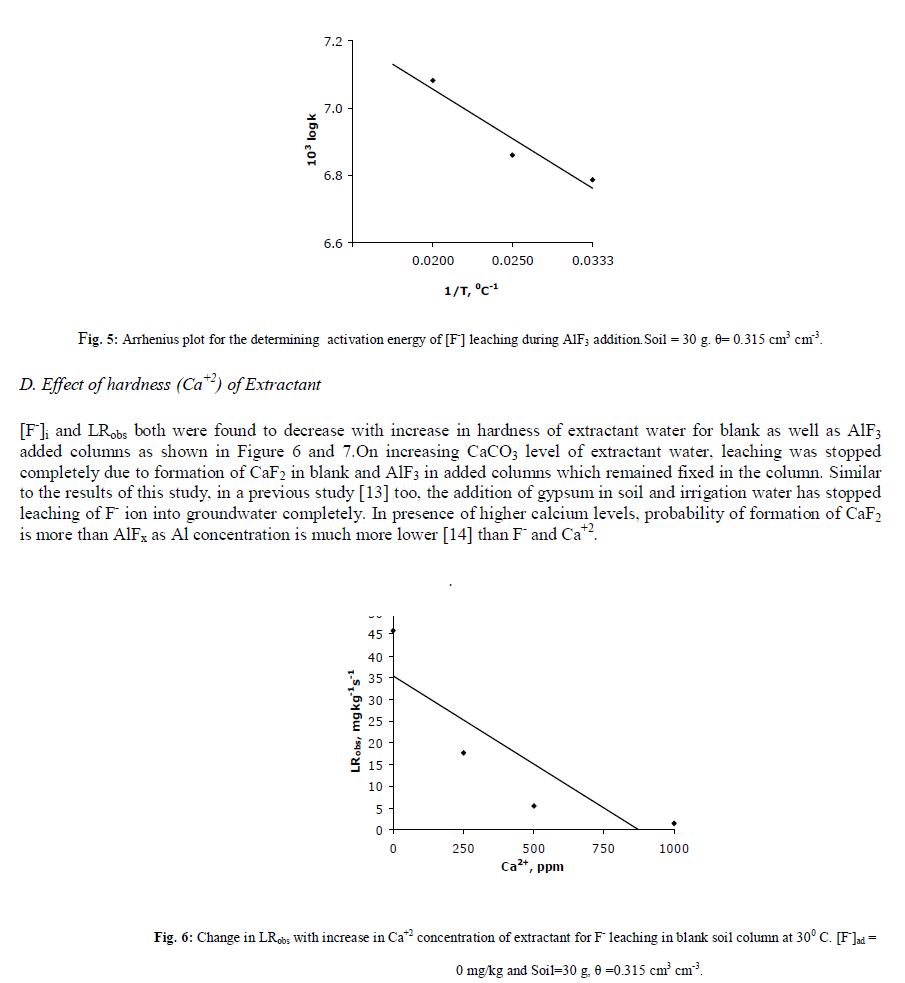 |
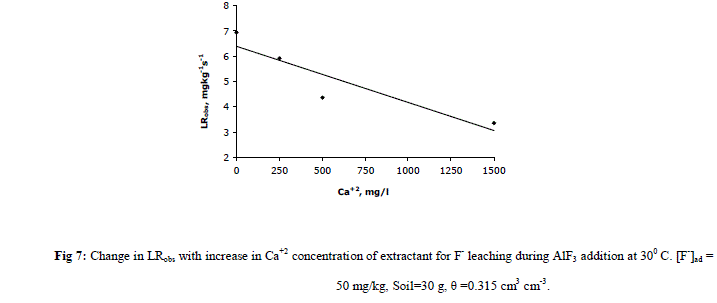 |
| E. Effect of Sodium Level of Extractant An increase in Na level in percolating water has increased [F-]complex thereby reducing [F-]i in blank as well as in AlF3 added columns. The decrease in [F-]i and LRobs in blank columns is attributed to the release of calcium and magnesium ions from the soil matrix during Na-Ca, Na-Mg exchanges which precipitate ionic fluoride as CaF2 and MgF2 in the column. When significant amount of Al3+ is added, a good concentration of free aluminium ions are also supposed to be present in soil water or matrix which may bind free F- present in the soil and convert it into unleachable form as cryolite (Na3AlF6) as both Al+3 and Na+ remain in very high concentrations in the column. The limiting nature of plot reflects that formation of fluoride complex depends upon soil type and size of the column along with added salt concentration. Our results reveal the fact that in soil rich in Sodium and Aluminium, fluoride leaching will be decreased due to formation of CaF2, AlF3 and Na3AlF6. Ionic fluoride is either not formed or even if, it is formed in small amount, immediately releases F- in soil-water which again gets precipitated as stable Al or Ca complexes limiting the leaching of fluoride from the column soil. |
| F. Effect of pH of Extractant [F-]i and LRobs are found to increase with increase in pH levels of extractant in AlF3 added columns. The order of increase in [F-]i and LRobs values are NH4OH > NaOH > KOH as shown in Table 3. Release of F- in the soil solution is possible during replacement of fixed fluoride by [OH-] ions which increases the concentration of leachable fluoride. The reason for maximum leaching during NH4OH addition is the formation of 4H-F bonds with ammonium ions to carry more fluorides which increases [F-]i and LRobs in comparison to Na+ and K+ [15]. |
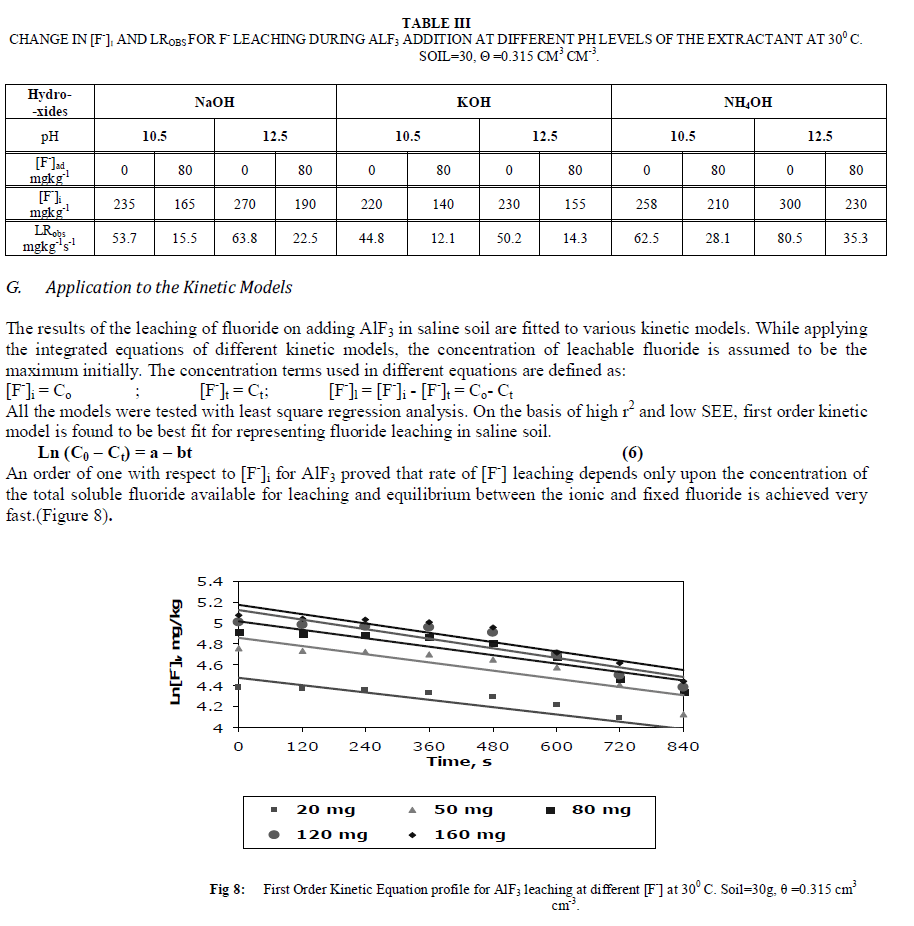 |
IV. CONCLUSION |
| The present study concludes that in fluoride rich saline soil with pH 8.2, leaching of F- will be decreased on adding AlF3. Ca+2 ions will impose negative effects on F- leaching while temperature will increase leaching. It is suggested that in saline soils of fluoride endemic areas, leaching of fluoride from soil to subsurface or groundwater can be controlled by increasing the hardness, SAR and aluminium content of the irrigation water or directly amending the soil with gypsum and water soluble aluminium salts eg AlCl3, Al2(SO4)3 etc. The result of this study of fluoride leaching from saline soils causing groundwater pollution can be applied to develop fluoride risk assessment models for fluoride pollution areas. |
References |
|