ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| A. S. Fouda*, G. Y. Elewady, K. shalabi, S. Habouba Department of Chemistry, Faculty of Science, Mansoura University, Mansoura, Egypt |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Anise extract, was investigated as green corrosion inhibitor of carbon steel in 1 M HCl solution using weight loss, potentiodynamic polarization, electrochemical impedance spectroscopy (EIS) and electrochemical frequency modulation (EFM) techniques. Surface morphology was tested using scanning electron microscope (SEM) and energy dispersive X-ray (EDX). The adsorption of the inhibitors on carbon steel surface was found to obey the Langmuir’s adsorption isotherm. The activation and adsorption parameters were calculated and discussed
Keywords |
| Anise extract, Carbon steel, Green Inhibitor, HCl, EDX, SEM |
INTRODUCTION |
| Carbon steel pipelines play an important role in transporting gases and liquids throughout the world [1]. Therefore, the prevention of metals used in petroleum field and industrial applications from corrosion is vital that must be dealt with especially in acid media [2, 3].The development of corrosion inhibitors is based on organic compounds containing Nitrogen, oxygen, sulfur atoms, and multiple bonds in the molecules that facilitate adsorption on the metal surface [4, 5]. Such compounds can absorb onto the metal surface and block the active surface sites, thus reducing the corrosion rate. Most of the natural products are nontoxic, biodegradable and readily available in plenty. Up to now, many plant extracts have been used as effective corrosion inhibitors for iron or steel in acidic media, such as Henna [6], NypafruticansWurmb[7], Zenthoxylumalatum[8], Damsissa[9], Menthapulegium[10], olive [11], Phyllanthusamarus[12]. Anise also called PimpinellaAnisum L., is an annual herb and a grassy plant with white flowers and small green to yellow seeds, which grows in Turkey, Iran, India, Egypt, and many other warm regions of the world. Its flavor has similarities with some other spices, such as star anise, fennel, and liquorice. Anise is sweet and very aromatic, distinguished by its characteristic flavor. The seeds, whole or powdered, are used in a wide variety of regional and ethnic confectioneries [13]. |
| This study aims to gain some insight into the corrosion of C-steel in HCl in the presence of anise as a corrosion inhibitor. The inhibitor effect of these naturally occurring biological molecules on the corrosion of C-steel in 1 M HCl was investigated by chemical and electrochemical techniques. |
II. EXPERIMENTAL |
2.1. Materials and reagents |
| Carbon Carbon steel strips (BDH grade) containing (weight %): 0.2 C, 0.024 P, 0.003 Si, 0.35 Mn, and rest Fe were used in this investigation. Coupons cut with 2 x 2 x 0.15 cm dimensions were used for weight loss measurements, whereas the specimens were embedded in epoxy resin leaving a working area of 1.0 cm2. The working surface was subsequently ground with 600 and 1200 grit grinding papers, cleaned by distilled water and ethanol. All chemicals used were of AR grade. The solutions (1 M HCl) were prepared by dilution of an analytical reagent grade 37% HCl with doubly distilled water. |
2.2. Preparation of plant extracts |
| The dried anise seeds sample were crushed to make fine powder. The powdered materials (250 g) were soaked in 500 ml of dichloromethane for 5 days and then subjected to repeated extraction with 5× 50 ml until exhaustion of plant materials. The extracts obtained were then concentrated under reduced pressure using rotary evaporator at temperature below 50°C. The dichloromethane evaporated to give solid extract that was prepared for application as corrosion inhibitor. |
| Chemical studies have demonstrated that the anise extract contain anethole, estragole, eugenol, pseudoisoeugenol, methylchavicol and anisaldehyde, coumarins, scopoletin, umbelliferon, estrols, terpene hydrocarbons, polyenes and polyacetylenes as the major compounds [14]. |
2.3. Weight loss measurements |
| The gravimetric method (weight loss) is probably the most widely used method of inhibition assessment [15 -17, 18- 21]. The simplicity and reliability of the measurement offered by the weight loss method is such that the technique forms the baseline method of measurement in many corrosion monitoring programmers [22]. Weight loss measurements were conducted under total immersion using 250 ml capacity beakers containing 50 ppm- 300 ppm test solution at 25-45°C maintained in a thermo stated water bath. The carbon steel coupons were weighed and suspended in the beaker with the help of rod and hook. For the effect of temperature on the inhibition efficiencies, all the tests were carried out in the temperature range25-45 °C. |
2.4. Electrochemical measurements |
| A three electrode electrochemical cell was used. The working electrode was carbon steel of surface area of 1.0 cm2. Before each experiment, the electrode was abraded using emery papers as before. After this, the electrode was cleaned ultrasonically with ethyl alcohol and washed by bidistilled water. All potentials were given with reference to the saturated calomel electrode (SCE). The counter electrode was a platinum plate of surface area of 1 cm2. Polarization measurements were carried out using a using Gamry Potentiostat/Galvanostat/ZRA (model PCI4/750) with a Gamry framework system based on ESA400 measurements; computer was used for collecting data. Echem Analyst 5.5 Software was used for plotting, graphing and fitting data. The working electrode was immersed in the test solution for 40 min to reach the open circuit potential was reached (the carbon steel used in the polarization measurements was identical to that used in the weight loss measurements).After that the working electrode was polarized in both cathodic and anodic directions. For checking the accuracy of the calculated slopes, the values were compared with the obtained data from the software calculations accompanied with the potentiostat electrochemical impedance spectroscopy (EIS) measurements were carried out as described elsewhere [23]. A small alternating voltage perturbation (5 mV) was imposed on the cell over the frequency range of 100 kHz to 0.1 Hz at open circuit potential and at 25°C. Each experiment was repeated for at least three times to check the reproducibility. EFM carried out using two frequencies 2 and 5 Hz. The base frequency was 1 Hz. In this study, we use a perturbation signal with amplitude of 10 mV for both perturbation frequencies of 2 and 5 Hz. |
2.5. Surface morphology |
| For morphological study, surface features (2.0 cm x 2.0 cm x 0.15 cm) of carbon steel were examined before and after exposure to 1.0 M HCl solutions for 4 hour with and without inhibitor. JEOL JSM-5500 scanning electron microscope was used for this investigation. |
III. RESULTS AND DISCUSSION |
3.1. Weight loss measurements |
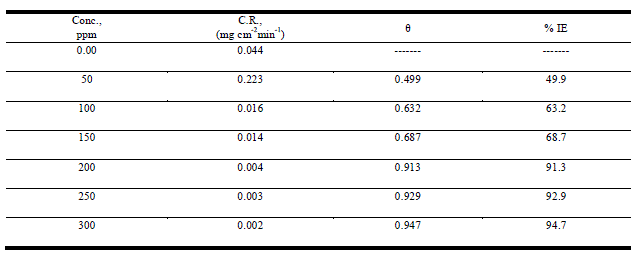
| Weight loss studies were carried out at five different temperatures 25-45°Cand the inhibition efficiency (IE%) values calculated are presented in Table 1. From the Tables, it is noted that the IE% increases steadily with increasing concentration of the inhibitor. The inhibition efficiency (IE%) surface coverage (θ) were calculated by the following expression: |
| % IE = θ x 100 = [1 – ( Δminh/ Δmfree)] ×100 (1) |
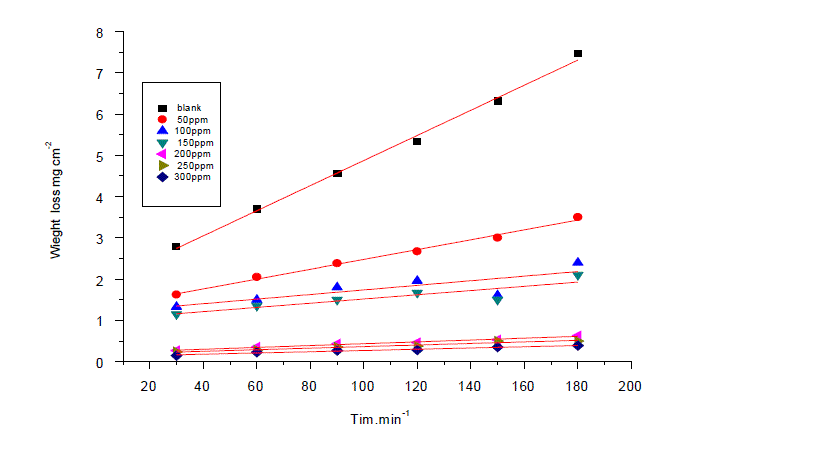
| whereΔmfree and Δminh are the weight losses per unit area in the absence and presence of inhibitor, respectively. Figure1 represents the dependence of inhibitor concentration for improved protection. The observed inhibition action of the anise extract could be attributed to the adsorption of its components on C-steel surface. The formed layer, of the adsorbed molecules, isolates the metal surface from the aggressive medium leading to decreasing the corrosion rate. |
3.2. Polarization curves |
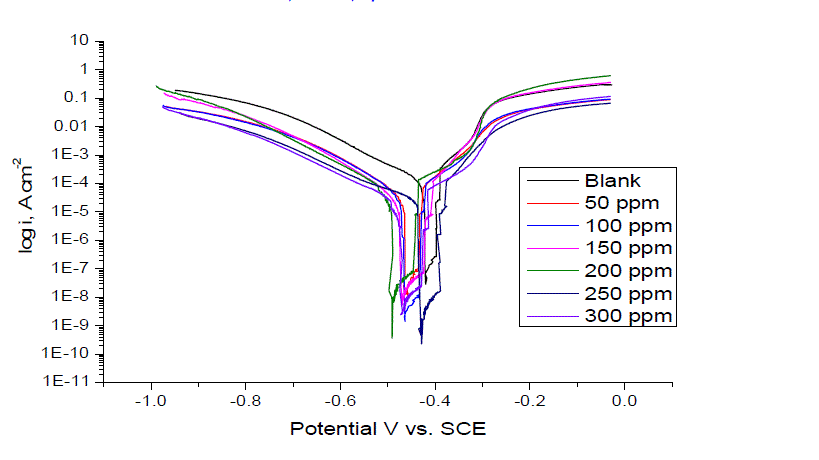
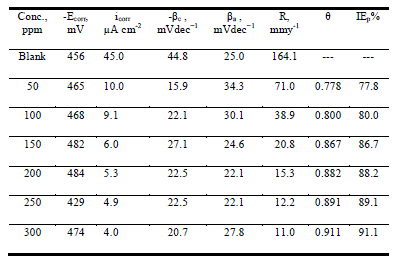
| Figure 2 shows the anodic and cathodic polarization curves of carbon steel in 1.0 M HCl solution, without and with anise extract at 25 ± 1°C after immersion for 40 min. It is observed that both the cathodic curves and anodic curves show lower current density in the presence of the Anise extract; the electrochemical parameters including: corrosion potential (Ecorr), corrosion current density (Icorr), cathodic and anodic Tafel slopes (βa, βc), of the corrosion process are listed in Table 2 in the presence of the anise extract, the Tafel slopes decreased to more negative potentials and increased to more positive potentials relative to the blank curve. The inhibition efficiency and surface coverage were calculated from the corrosion current densities |
| IE% = θ × 100 = [1 – (i°corr/ icorr)] × 100 (2) |
| Where i°corr and icorr represent uninhibited and inhibited corrosion current densities, respectively. |
| The anise extract molecules adsorbed merely by blocking the reaction sites of iron surfaces lead to the reduction of surface area available for hydrogen evolution. Therefore, it could be concluded that the anodic iron dissolution and cathodic hydrogen evolution reaction were both inhibited by the Anise extract through merely blocking the reaction sites of carbon steel surface without affecting the anodic and cathodic reaction mechanism [24]. Also, this suggests the mixed behavior of the used inhibitors, i.e., mixed type inhibitors [25]. The irregular trends of βa and βc values indicate the involvement of more than one type of species adsorbed on the metal surface. |
3.3. Electrochemical impedance spectroscopy |
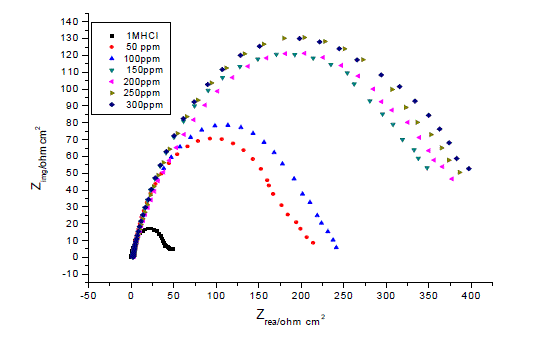
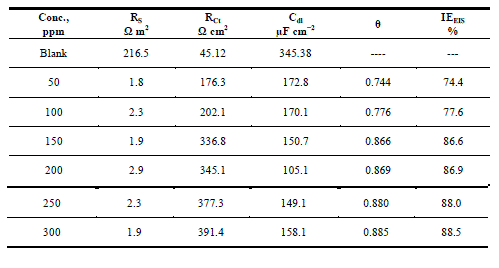
| Figures The corrosion behavior of carbon steel in 1.0 M HCl in the presence and absence of the anise extract was investigated using EIS at 25°C. Nyquist plots of carbon steel in 1.0 M HCl solution with and without various concentrations of anise extract are given in Fig. 3. As shown in Fig. 3, the capacitive loops are slightly depressed as semi-circular shapes because of the roughness and other in homogeneities of carbon steel surface resulting in a phenomenon called ‘‘dispersing effect’’ [26, 27].A capacitive loop arises from the time constant of the electric double layer and charge-transfer resistance, and an inductive loop appeared at Low-frequency (LF) values in the presence of Anise extract. The presence of the LF inductive loop may be attributed to the relaxation process obtained by adsorption the π-electrons of the conjugated systems O- on the electrode surface [28–31]. It may also be attributed to adsorption of inhibitor on the electrode surface [32] or to the re-dissolution of the passivated surface at low frequencies [33]. In other words, the inductive behavior at low frequency is probably due to the consequence of the layer stabilization by products of the corrosion reaction on the electrode surface (for example, [FeOH] and [FeH]) involving inhibitor molecules and their reactive products[34].Rs represent the charge transfer resistance. It measures the electron transfer across the surface, which is inversely proportional to the corrosion rate [35]. |
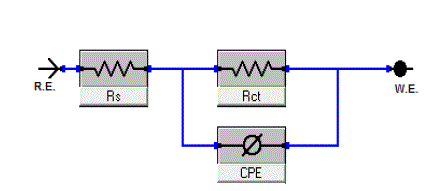
| The equivalent circuit depicted in Figure 8is employed to analyze the impedance spectra, where Rs represents the solution resistance, Rct denotes the charge-transfer resistance, and the constant phase element (CPE) instead of a pure capacitor represents the interfacial capacitance. The impedance of a CPE is described by the equation 3[36]: |
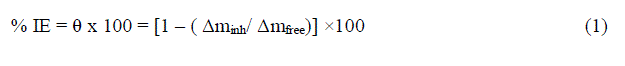 |
| The lower Cdl values Table.3 from the blank as the concentration of the inhibitor increases confirm the enhancement of adsorption of the inhibitor on the metal surface. The decrease in Cdl is attributed to an increase in thickness of the electronic double layer due to adsorption [43].The adsorption is due to the electronegative hetero atoms present in the organic constituents of Anise extract on the electropositive metal surface. All the electrochemical parameters clearly propose that the corrosion control depends on the concentration of the inhibitor as illustrated in Fig.3.The inhibition efficiencies, calculated from EIS results, show the same trend as those obtained from polarization measurements. The difference of inhibition efficiency from two methods may be attributed to the different surface status of the electrode in two measurements. EIS were performed at the rest potential, while in polarization measurements the electrode potential was polarized to high over potential, non-uniform current distributions, resulted from cell geometry, solution conductivity, counter and reference electrode placement, etc., will lead to the difference between the electrode area actually undergoing polarization and the total area [44]. |
3.4. Electrochemical Frequency Modulation Technique (EFM) |
| The EFM is a nondestructive corrosion measurement like EIS; it is a small signal ac technique. Unlike EIS, however, two sine waves (at different frequencies) are applied to the cell simultaneously. The great strength of the EFM is the causality factors which serve as an internal check on the validity of the EFM measurement [45]. With the causality factors the experimental EFM data can be verified. The results of EFM experiments are a spectrum of current response as a function of frequency. The spectrum is called the intermodulation spectrum. The spectra contain current responses assigned for harmonical and intermodulation current peaks. The larger peaks were used to calculate the corrosion current. The inhibition efficiencies, IE% calculated from Equation 6 increase with increasing the studied inhibitor concentrations: |
| IE% = θ × 100 = [1 – (i°corr/ icorr)] × 100 (6) |
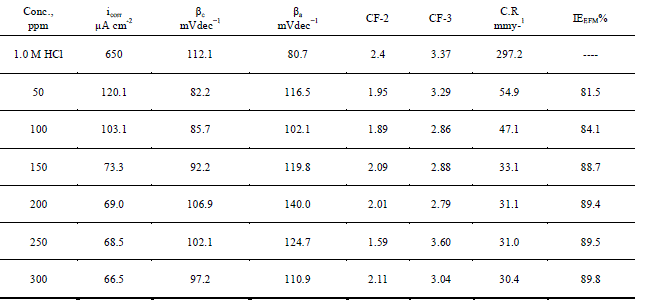
| The two frequencies may not be chosen at random. They must both be small, integer multiples of a base frequency that determines the length of the experiment. Intermodulation spectra obtained from EFM measurements were constructed for iron 1.0 M HCl solutions as a function of Anise extract concentration at 25 0C. Each spectrum is a current response as a function of frequency; data not shown here. Corrosion kinetic parameters, namely corrosion current density (icorr, Tafel constants (βa,βc) and causality factors (CF-2, CF-3) were listed Table4as a function of Anise extract concentration at 250C.data can be verified. The causality factors in Table 4, which are very close to theoretical values according to the EFM theory, should guarantee the validity of Tafel slopes and corrosion current densities. The standard values for CF-2 and CF-3 are 2.0 and 3.0, respectively [46, 47]. |
3.5. Adsorption isotherm |
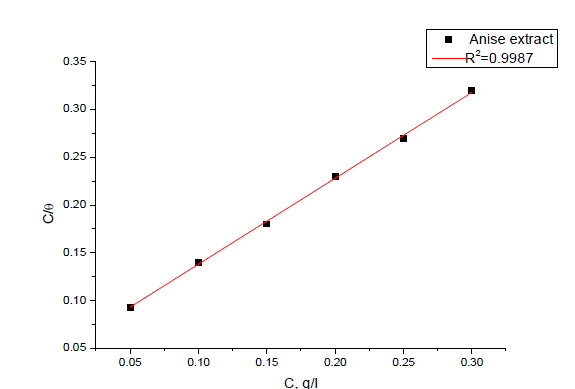
| The mode and interaction degree between an inhibitor and a metallic surface have been widely studied with the application of adsorption isotherms. The adsorption of an organic molecule occurs because the interaction energy between an inhibitor and a metallic surface is higher than that between water molecules and metallic surface [48, 49]. To obtain the adsorption isotherms, the degree of surface coverage (θ) obtained from weight loss method was determined as a function of inhibitor concentration. The values of θ were then plotted to fit the most suitable model of adsorption [50].Attempts were made to fit experimental data to various isotherms including Frumkin, Langmuir, Temkin, Freundlich, isotherms. By far the results were best fitted by Langmuir adsorption isotherm equation [51]: |
| C/ θ = 1/K+C (7) |
| The adsorption equilibrium constant (K) is related to the standard free energy of adsorption (ΔG°) as shown the following equation [52]: |
| K= 1/55.5 x (G° ads /RT) (8) |
| Where R is the gas constant (8.314 J K mol-1), T the absolute temperature (K), and the value 55.5 is the concentration of water in solution expressed in M. |
| TheΔG° values are also given in Table 5.The negative values of ΔG° indicate that the adsorption of inhibitor molecule on steel surface is a spontaneous process. Generally, values of ΔG° up to 20 kJ mol-1are consistent with the electrostatic interaction between the charged molecules and the charged metal (physical adsorption) while those more negative than 40 kJ mol-1involve sharing or transfer of electrons from the inhibitor molecules to the metal surface to form a coordinate type of Bond (chemisorptions) [53] kJ mol-1; probably means that the adsorption of either Anise extract on carbon steel surface involves both physical adsorption and chemical adsorption. |
3.6. Kinetic-thermodynamic corrosion parameters |
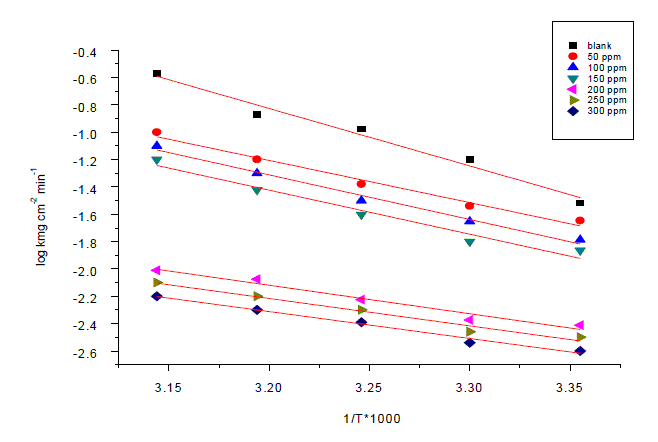
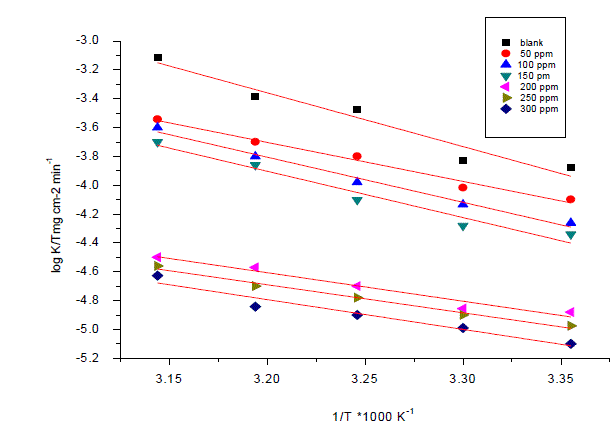
| Weight loss method was carried out at different temperature (25°C–45°C) in the presence of different concentration of Anise extract. It has been found that he corrosion rate decreases with the increase in temperature for Anise extract (Table 6). The corrosion rate of carbon steel in the absence of anise extract increased steeply from 25 to 45°Cwhereas; in the presence of anise extract the corrosion rate decreased slowly. The inhibition efficiency was found to increase with temperature. The corrosion parameter in the absence and presence of extract in the temperature range 25–45°C has been summarized in Table 6.The apparent activation energy (Ea) for dissolution of carbon steel in 1M HCl was calculated from the slope of plots by using Arrhenius equation: |
| Log k=-Ea/2.303RT+logA (9) |
| Where k is rate of corrosion, Ea Vis the apparent activation energy is the universal gas constant, T is absolute temperature and A is the Arrhenius pre-exponential factor. By plotting log k against 1/T the values of activation energy (Ea) has been calculated (Ea = (slope) 2.303 · R) (Fig. 7). Activation energy for the reaction of carbon steel in 1M HCl decreases in the presence of extract (Table7). The decrease in activation energy Ea indicates the formation of chemical bonds were strengthen by increasing the temperature. However, the extent of the rate increment in the inhibited solution is higher than that in the free acid solution. Therefore, the inhibition efficiency of the ÃËônise extract increases markedly with increasing temperature. This result supports the idea that the adsorption of extract components on the steel surface may be chemical in nature. Thus, as the temperature increases the number of adsorbed molecules increases leading to an increase in the inhibition efficiency. The obtained results suggest that anise extract inhibits the corrosion reaction by decreasing its activation energy. This could be done by adsorption on the steel surface making a barrier for mass and charge transfer. However, such types of inhibitors perform a good inhibition at high temperature with considerable increase in inhibition efficiency at elevated temperatures [54]. Moreover, the relatively low value of activation energy in presence of anise extract suggests a chemical adsorption process. |
| The values of change of entropy (ΔS*) and change of enthalpy (ΔH*) can be calculated by using the formula: |
| k = (RT/Nh) exp (ΔS*/R) exp (ΔH*/RT) (10) |
| Where k is rate of corrosion, h is Planck’s constant, N is Avogadro number, ΔS* is the entropy of activation, and ΔH* is the enthalpy of activation. A plot of log (k/T) vs. 1/T (Fig. 8) should give a straight line, with a slope of (ΔH*/2.303R) and an intercept of [log (R/Nh)+ΔS/2.303R], from which the values of ΔS and ΔH can be calculated (Table .7).The negative value of ΔS* (Table .7) for both the inhibitors indicates that activated complex in the rate determining step represents an association rather than a dissociation step, meaning that a decrease in disorder takes place during the course of transition from reactant to the activated complex [55] The negative sign of ΔH* indicates that the adsorption of inhibitor molecules is an exothermic process. Generally, an exothermic process signifies either physisorption chemisorption’s or a combination of both. |
3.7. Surface analysis |
3.7.1. Surface analysis by SEM |
| Fig.9 shows an SEM photograph recorded for C-steel samples Polished (A) and exposed for 4 h in 1M HCl solution without (B) and with 300ppm of Anise extract at 25C0. A photograph of the polished carbon steel surface before immersion in 1 M HCl solution is shown in Fig. 5a. The photograph shows the surface was smooth and without pits. The SEM micrographs of the corroded carbon steel in the presence of 1 M HCl solution are shown in Fig. 9b. The faceting seen in this figures was a result of pits formed due to the exposure of carbon steel to the acid. The influence of the inhibitor addition 300ppm on the carbon steel in 1 M HCl solution is shown in Fig. 9c. The morphology in Fig. 9c shows a rough surface, characteristic of uniform corrosion of C-steel in acid, as previously reported [56], that corrosion does not occur in presence of inhibitor and hence corrosion was inhibited strongly when the inhibitor was present in the hydrochloric, and the surface layer is very rough. In contrast, in the presence of 0.300ppm of Anise extract, there is much less damage on the steel surface, which further confirm the inhibition action. Also, there is an adsorbed film adsorbed on carbon steel surface (Fig. 9c).In accordance, it might be concluded that the adsorption film can efficiently inhibits the corrosion of carbon steel. |
3.7.2. Surface examination by EDX |
| EDX survey spectra were used to determine which elements were present on the electrode surface before and after exposure to the inhibitor solution. Fig. 10a, b and c show EDX spectra of the carbon steel surface after immersion in 1 M HCl, for a period of 4 h, in absence and presence of 300 ppm of Anise extract, respectively. The EDX spectra show the characteristics peaks of some of the elements constituting the steel sample after 4 h immersion in 1 M HCl without inhibitor. From table (8) in presence of 300 ppm of Anise extract, the EDX spectra show additional lines of carbon and oxygen, due to the adsorbed layer of inhibitor that covered the carbon steel surface. In addition, the Fe peaks are considerably suppressed relative to uninhibited steel surface sample. This suppression of the Fe lines occurs because of the overlying inhibitor film. These results confirm those from weight loss, polarization and EIS measurements, which suggest that a protective film formed over the metal surface, and hence retarded both anodic and cathodic reactions [57]. |
3.7 Mechanism of Corrosion inhibition |
| The adsorption process is affected by the chemical structures of the inhibitors, the nature and charged surface of the metal and the distribution of charge over the whole inhibitor molecule. Thermodynamic parameters showed that the adsorption of anise extract on the carbon steel surface 1 M HCl solution is chemical than physical one. Chemical adsorption of anise extract arises from the donor acceptor interactions between free electron pairs of hetero atoms and π-electrons of multiple bonds as well as phenyl group and vacant d orbital's of iron [58, 59]. It has been reported that the adsorption of heterocyclic compounds occurs with the aromatic rings sometimes parallel but mostly normal to the metal surface. The inhibition action of anise extract does not occur by the simple blocking at the surface of carbon steel, especially at high temperature. This might be attributed to the different adsorption capacities of the anise extract on the carbon steel surface at different temperatures. It has been studied that with the increase in temperature, the desorption effect of anise extract carbon steel surface decreased. Some of the hydrophilic groups with positively charged atoms (O+) desorbed from the surface of carbon steel and did more work to prevent the H+from getting nearer to the metal surface. Therefore, Anise extract preferentially inhibited the cathodic corrosion process at high temperature. |
CONCLUSIONS |
| From the overall experimental results the following conclusions can be deduced: |
| 1. The Anise extract shows good performance as corrosion inhibitor 1 M HCl. |
| 2. Potentiodynamic polarization measurements, EFM showed that the Anise acts as mixed-type inhibitor. EIS measurements also indicate that the inhibitor increases the charge transfer resistance and show that the inhibitive performance depends on adsorption of the molecules on the metal surface. |
| 3. The inhibition efficiencies determined by weight loss, potentiodynamic polarization and EIS techniques are in reasonably good agreement. |
| 4. The Anise extract inhibits the corrosion by getting adsorbed on the metal surface following Langmuir adsorption isotherm. |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
References |
|