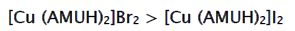e-ISSN: 2319-9849
e-ISSN: 2319-9849
1Department of Chemistry, Mizoram University, Aizawl, Mizoram, India
2Department of Chemistry, Nagaland university, Lumami, Nagaland, India
Received date: 07/06/2013; Revised date: 18/06/2013; Accepted date: 25/06/2013
Visit for more related articles at Research & Reviews: Journal of Chemistry
Our work has done to measure conductivity of copper (II) ion complexes in aqueous medium at different temperatures ranging from 283 to 303K. The limiting molar conductance values have been calculated at molar concentrations varying from 1.1 to 0.569 x 10-3 M. The conductance data in all the cases have been analyzed by Shedlovsky Technique to obtain limiting molar conductance (Λ0) and ion association constant (KA) values for the electrolytes. It is observed that the limiting equivalent conductance increased linearly with the increased in temperature and the association constant values decreased with rise in temperature. The limiting molar conductance and ion association constant values for bis-1-amidino-O-methylurea copper (II) bromide are found to be higher than those in bis-1-amidino-O-methylurea copper (II) iodide. Thermodynamic parameters (i.e., ΔG0, ΔH0, ΔS0) are estimated from the temperature dependence of the ion association constant. The selected two metal complexes show ion-pair association within experimentally different temperatures which are supported by the negative value of ΔG0. The positive values of ΔS0 and positive values of ΔH0 indicate that the ion association process occurred spontaneously as well as endothermic at all respective temperatures. The association constant values for both complexes are determined and found in the order bis-1-amidino-O-methylurea copper (II) bromide > bis-1-amidino-O-methylurea copper (II) iodide.
Electrical conductivity, Shedlovsky Technique, Association constant, Thermodynamic parameters, Metal complexes.
The partial association of oppositely charged ions in electrolyte solution to form distinct chemical species called ion pairs. An ion pair is a physical entity with no specific chemical interactions between the ions. The ions of the ion pair move together as a single unit and are held together by electrostatic forces of the coulomb type acting over the short distances that the ions are apart in the ion pair [1]. These Coulombic forces impose a certain degree of cohesion on the unit and this is sufficiently great to overcome the tendency for normal thermal motion to cause the ions to move around as separate particles each with its own translational degrees of freedom. For two oppositely charged ions to stick together to form an ion pair, it is necessary that they should be closed enough for the Coulombic attraction energy to overcome the thermal energy that scatters them apart [2]. In any solution of an electrolyte there is always the possibility that the ions of the electrolyte might not be fully dissociated in solution. Ion pairing results when the electrostatic interaction between two oppositely charged ions become sufficiently large for the two ions to move around as one entity, the ion-pair. The extent of association into ion-pairs depends on many factors [3], with the most important being the nature, charges and sizes of the ions, the characteristics of the solvent and the temperature. The measurements of electrical conductivities of dilute solutions of salts or complexes are considered to be one of the important methods for studying the ion-pair or multiple ion association not only in aqueous solution but also in non-aqueous or mixed ones [4,5]. Also conductivity measurements were used to evaluate the hydration free energy of some electrolytic solutions and to study the nature of the solute-solvent interactions [6-8]. In aqueous electrolytic solutions the most important role is played by ion hydration and ion association, which is essentially depend not only on the concentration of dissolved substance and on chemical nature of ions, but on the external parameters (p and T). Most properties of electrolyte solutions depend on the ability of solvent and solute to interact, and hence on the nature of the complex ion formation.
The present work reports the comparative studies of conductometric properties and thermodynamic properties of bis-1-amidino-O-methylurea copper (II) bromide and bis-1-amidino-O-methylurea copper (II) iodide in water at different temperatures ranges 10-300C. The data were analyzed by using Shedlovsky extrapolation method. We have also investigated the effect of temperature on limiting molar conductance and association constant for the selected compounds which have been supported by the calculated values of standard thermodynamic parameters. These results were discussed in terms of the association constants and also approach of Gibbs energy relationship [9] can be applied to the association process at various temperatures to discuss the thermodynamic features of metal-ion complexes. The KAS and Λ0 have been evaluated in these solvents at the experimental temperatures. The thermodynamic parameters viz., ΔH0, ΔS0 and ΔG0 for the formation have been studied from the values of ion association constant at various temperatures.
The measurements of electrical conductivities of dilute solution of salts or complexes are considered to be one of the important methods for studying the ion pair or multiple-ion association not only in aqueous solutions but also in non-aqueous or mixed ones [10]. The limiting molar conductance and the association constants of the complex ion with anions were calculated by using Shedlovsky method [11]. The electrical conductivities were measured by Orion Star A112 Conductivity Benchtop Meter with a dip type immersion conductivity epoxy 2 Cell (K=1.0) was used. The solutions of different concentrations (1.1-0.569x 10-3M) were carefully prepared by dissolving requisite amount of the sample in conductivity water (i.e., double distilled water) of specific conductance (< 3x10-6Scm-1). Conductivity measurements were carried out over the temperature range 10-300C. All the dielectric constants and viscosities were obtained from literature [12,13]. The temperature control in the ranges 10 -300C were made by using water bath and circular –Model D8-G of HAAKE Mess-Technik Gmbtlu. Co. Germany. The measurements of weights were done by using a METTER Balance, Model-AE 260, Delta Range. All calculations were done on IBM-PC-AT/386 using a basic programmed.
The experimental data of conductance measurements were analyzed by using Shedlovsky equation. Shedlovsky equation is given by
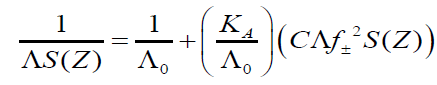 .........................(1)
.........................(1)
Where Λ is molar conductance at a concentration C (g.mol.dm-3), Λ0 is the limiting molar conductance and KA is the observed association constant. The other symbols are given by Z and λ are the valence and conductance of the ions respectively, excluding their signs; D is the dielectric constant of the medium, η the viscosity (c.p.). The degree of dissociation (τ) is related to S(Z) by the equation,
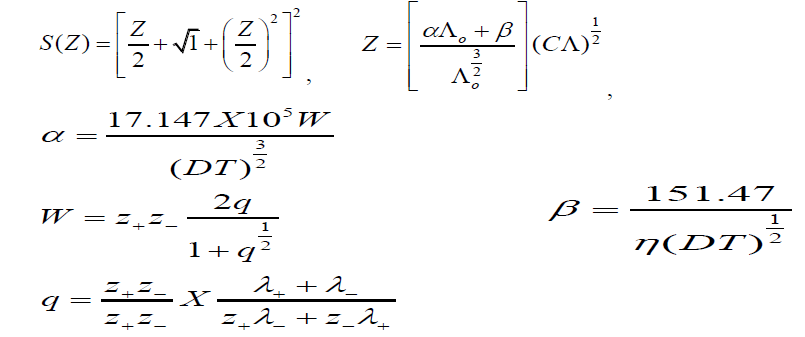
z and λ are the valence and conductance of the ion respectively, excluding their signs, D is the dielectric constant of the medium, η the viscosity (c.p). The degree of dissociation (τ ) is related to S (Z) by the equation:
τ = ΔS (Z)/Δ0
f± is the activity coefficient of the free ions and was calculated as
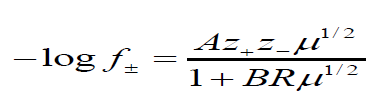 (2)
(2)
Where,
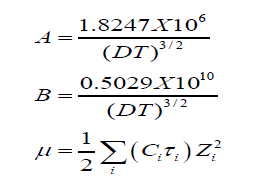
R is the maximum centre to centre distance between the ions in the ion-pair. There exists at present no method of determining the value of R precisely. In order to treat the data in our system the R value is assumed to be R = a+d, where a, the sum of crystallographic radii of the ions, is approximately equal to 5A0 and d (A0) is given by:
d = 1.183(M/ρ)1/3 (3)
Where, M is the molecular weight of the solvent and ρ the density of the solution.
An initial value of Λ0 was obtained by least square method (Λ) and concentration C were introduced as input in computer programme. The mean activity coefficient f was determined by equation (2) for the above chosen complex salts. From the linear plot of 1/ΛS (Z) versus C Λf±2 S(Z); Λ0 and KA was evaluated from the intercept 1/ Λ0 and the slope KA /Λ02 respectively . The procedure was repeated using these new values of Λ0 and KA. All calculations were carried out by IBM-PC.
The Change of free energy for the association process (ΔG0) was calculated from the equation:
ΔG=-2.303RTlog10KA (4)
The enthalpy change of association (ΔH0) was obtained from the slope of log KA vs 1/T. The change of association entropy (ΔS0) was calculated from the Gibbs- Helmholtz equation:
ΔS0=(ΔH0-ΔG0)/T (5)
The Change of free energy for the association process (ΔG0) was calculated from the equation:
ΔG=-2.303RTlog10KA (6)
The enthalpy change of association (ΔH0) was obtained from the slope of log KA vs 1/T. The change of association entropy (ΔS0) was calculated from the Gibbs- Helmholtz equation:
ΔS0=(ΔH0-ΔG0)/T (7)
From tables 1 & 2, the values of Λ0 for the title electrolytes increase invariably with increase in temperature in aqueous medium, indicating less salvation or higher mobility of ions [14]. This is due to the fact that the increased thermal energy results in bond breaking and leads to higher frequency and higher mobility of ions [15]. Values of Λ0 for [Cu(AMUH)2]Br2 are always greater than those values of the complex [Cu(AMUH)2]I2 is most prone to the variation in vibrational, rotational and translational with temperature. The values of KAs are formed in increasing order [Cu (AMUH)2] Br2 > [Cu(AMUH)2]I2. This is because the size of the anion for bromide is smaller than the iodide ion. Both values of KAs for these two salts decrease with rise in temperature(Fig.1). This decrease in KA is due to the fact that the increase thermal energy results in greater bond breaking and variation in vibrational, rotational and translational energy of molecule leads to dissociation of molecules [16]. The free energy change (ΔG0) for association is calculated from the relation ΔG0=-RT lnKA. The heat of association ΔH0 is obtained from the slope of the plot of log KA vrs 1/T (Fig. 2). The entropy change (ΔS0) is calculated from the Gibbs-Helmholtz equation, ΔS0=(ΔH0-ΔG0)/T. The values of thermodynamic functions are given in table 3 and table 4. The positive values of ΔH0 indicate that ion association processes are endothermic at all temperatures [17].
Out of these two salts, negative values of ΔG0 is more in bis-1-amidino-O-methylurea copper(II) bromide salt and this complex is more favored in ion pair formation. A positive entropy change is broken when association takes place leading to an increase in the degree of disorderliness [18].
The limiting molar conductance (Λ0) increased linearly with the increase in temperature and the association constant (KA) values decrease with rise in temperature. Both the reactions are endothermic in nature which is determined by positive values of ΔH0. The chosen metal complexes show ion – pair association within experimental different temperatures ranges; which is supported by negative values of ΔG0. Out of these two salts, negative values of ΔG0 is more in bis- 1-amidino-O-methylurea copper(II) bromide salt and this complex is more favored in ion pair formation. The KA values for bis-1-amidino-O-methylurea copper (II) bromide are better than bis-1-amidino-O-methylurea copper (II) iodide and found in the order: