ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Md Koushik Chowdhury1, Anuj Srivastava2, Dr.Neeraj Sharma3, Dr.Shiru Sharma4 Research Scholar, School of Biomedical Engineering, IIT-(BHU), Varanasi, Uttar Pradesh, India1 Research Scholar, School of Biomedical Engineering, IIT-(BHU), Varanasi, Uttar Pradesh, India2 Associate Professor, School of Biomedical Engineering, IIT-(BHU), Varanasi, Uttar Pradesh, India3 Assistant Professor, School of Biomedical Engineering, IIT-(BHU), Varanasi, Uttar Pradesh, India4 |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
This paper describes the critical and necessary points required for the design and development of the noninvasive blood glucose monitoring device. The lifestyle quality of the Diabetic patients will improve with the noninvasive technology due to its tight monitoring over the fluctuations in higher or lower blood glucose levels. Noninvasive blood glucose detecting techniques will provide increased patient compliance with pain free, low cost blood glucose monitoring. Optically based noninvasive techniques have brought improvement in this area. Various noninvasive methods have been emerged for noninvasive blood glucose detections, present paper describes their methods, significance and limits. All these technologies are based on the selectivity of the analytical information, instrumental signal to noise ratios, specific properties of the measurement sites and instrumental efficacy for the blood glucose specific signal acquisitions.
Keywords |
||||
| Noninvasive, Blood Glucose, signal to noise ratio, hyper and hypoglycemia | ||||
INTRODUCTION |
||||
| Diabetes mellitus is a metabolic disorder in which our body is unable to regulate the level of glucose in the blood. As of year 2000 at least 171 million people worldwide suffer from diabetes, expected 366 million in 2030[1]. In order to maintain the blood glucose concentration the diabetic patients need to check his/her blood glucose level at regular intervals by puncturing (invasive method) the body sites applicable. Thus puncturing body sites for fresh blood samples provides mental agony to the patients and is painful also. As a result there is initiation of patient’s non-compliance towards regular monitoring of blood glucose level. Therefore diabetes prevention faces major setback. | ||||
| The development of a noninvasive method would considerably improve the quality of life for diabetic patients, facilitate their compliance for glucose monitoring, and reduce complications and mortality associated with this disease. Further, with the availability of non invasive blood glucose meter it will become possible to continuously monitor the blood glucose level. Hence, noninvasive and continuous monitoring of glucose concentration in blood and tissues is one of the most challenging and exciting applications of optics in medicine in present day scenario. This paper is organized as follow: Section I gives the introduction about the occurrence of diabetes mellitus & non invasive blood glucose detection techniques. Section II describes the current optical based approaches and methods. Section III explains the challenges & countermeasures in effective noninvasive glucose measurements. Section IV shows the principle of measurement. Section V describes the proposed methodology. Section VI concludes the paper and followed by the references. | ||||
II.CURRENT OPTICAL BASED APPROACHES AND METHODS |
||||
| A. Polarization changes: It is based on the phenomenon that occurs when polarized light transverses a solution containing optically active solutes (such as chiral molecules): the light, in fact, rotates its polarization plane by a certain angle, which is related to the concentration of the optically active solutes [2]. Glucose is a chiral molecule, and its light rotation properties have been known for a long time. Indeed, investigation of the polarization changes induced by glucose is reported to be the first proposed non-invasive technique for glucose measurement in humans [3]. Advantage of Polari-metric technique is that this can make use of visible light, easily available. Moreover, the optical components can be easily miniaturized [4]. However, Limitations are: this technique is sensitive to the scattering properties of the investigated tissue, since scattering depolarizes the light. As a consequence, skin cannot be investigated by polarimetry, since it shows high scattering due in particular to the stratum corneum [5]. Moreover, the specificity of this technique is poor, since several optically active compounds are present in human fluids containing glucose, such as ascorbate and albumin. However, specificity can be partially improved by using multiple light wavelengths. Other general sources of errors are variations in temperature and pH of the solution. Some error sources specific of the investigated site are reported. | ||||
| B. Optical Coherence Tomography: The optical coherence tomography (OCT) is based on the use of a low coherence light, such as a super luminescent light, an interferometer with a reference arm and a sample arm, a moving mirror in the reference arm and a photo detector to measure the interferometric signal [6]. Light backscattered from tissues is combined with light returned from the reference arm of the interferometer, and the resulting interferometric signal is detected by the photo detector. What is measured is the delay correlation between the backscattered light in the sample arm and the reflected light in the reference arm [2]. Moving the mirror in the reference arm of the interferometer allows scanning the tissues up to a depth of about 1 mm. By moving the mirror into the sample arm scanning of the tissue surface is obtained. Therefore, this technique has unique capability of in-depth and lateral scanning to obtain two-dimensional images with high resolution. Tissue scattering properties are highly dependent on the ratio of the refractive index of scattering centers (cellular components, proteins, etc.) to the refractive index of the interstitial fluid. Advantage are: an increase of glucose concentration in the interstitial fluid causes an increase in its refractive index, thus determining a decrease in refractive index mismatch, and hence of the scattering coefficient. Therefore, from the OCT data, generated by the backscattered light, it is possible to get an estimation of glucose concentration in the interstitial fluid. [7]. Limitations of OCT technique can be sensitive to motion artifacts. Moreover, although little changes in skin temperature have negligible effects, changes of several degrees have a significant influence on the signal [8]. There is currently no clear indication that this technique has advantages compared to other scattering based techniques [6]. | ||||
| C. Fluorescence technology: This technique is based on the generation of fluorescence by human tissues when excited by lights at specific frequencies. In the case of glucose, one study demonstrated that when a glucose solution is excited by an ultraviolet laser light at 308 nm, fluorescence can be detected at 340, 380, 400 nm, with maximum at 380 nm [2]. It was also proved that fluorescence intensity was dependent upon glucose concentration in the solution. Advantage: Light in the visible spectrum can be used and more adequate for studying fluorescence of tissues. Limitations: In tissues, the use of ultraviolet light could lead to strong scattering phenomena, in addition to fluorescence. Moreover, even when using different wavelengths, the fluorescence phenomenon can depend not only on glucose, but on several parameters, such as skin pigmentation, redness, epidermal thickness [9]. | ||||
| D. Ocular spectroscopy: This technique is based on the use of a special contact lens where a hydro gel has been added. A hydro gel wafer with 7 micrometer of thickness based on boronic acid derivatives was bonded to the lens. The boronic acid derivative is able to create reversible covalent bonds with glucose, and the phenomenon is influenced by the glucose concentration in tears. When the lens is illuminated by a light source (such as a laser light), the reflected light changes its wavelength (i.e. its color) depending on the entity of the binding phenomenon, which is related to tear glucose concentration. The light color changes can be detected by a spectrometer [10]. Advantage The preferential site for this technique is the eye, and, more specifically, the aqueous humor beneath the cornea [11]. Cornea has in fact low scattering properties, since it does not have the stratum corneum. Limitations: There is a delay between glucose in blood and in tears. Moreover, the use of contact lens, though it can be considered a non-invasive approach, may be uncomfortable to some subjects. | ||||
| E. Infrared spectroscopy: Near infrared spectroscopy (NIR) and mid infrared spectroscopy (Mid IR). | ||||
| Near infrared spectroscopy (NIR): The light focused on the body is partially absorbed and scattered, due to its interaction with the chemical components within the tissue. Glucose concentration could be estimated by variations of light intensity both transmitted through a glucose containing tissue and reflected by the tissue itself. Transmission or reflectance (localized or diffuse) of the light can be measured by proper detectors. Advantage: Near infrared (NIR) spectroscopy is based on focusing on the body a beam of light in the 750–2500 nm spectrum [12]. NIR spectroscopy allows glucose measurement in tissues in the range of 1–100 mm of depths, with a decrease in penetration depth for increasing wavelength values. Limitations: The absorption coefficient of glucose in the NIR band is low and is much smaller than that of water by virtue of the large disparity in their respective concentrations. Thus, in the NIR the weak glucose spectral bands only overlap with the stronger bands of water, but also of hemoglobin, proteins and fats. As regards the scattering coefficient, the effect of a solute (like glucose) on the refractive index of a medium is non-specific, and hence it is common to other soluble analytes. | ||||
| Furthermore, physical and chemical parameters, such as variation in blood pressure, body temperature, skin hydration, triglyceride and albumin concentrations may interfere with glucose measurement [5]. Errors can also occur due to environmental variations, such as changes in temperature, humidity, carbon dioxide and atmospheric pressure. | ||||
| Mid-infrared spectroscopy (Mid-IR): Mid-infrared (Mid-IR) spectroscopy is based on light in the 2500–10,000 nm spectrums [2]. The physical principle is similar to that of NIR. When compared to NIR, however, due to the higher wavelengths, Mid-IR exhibits decreased scattering phenomena, and increased absorption. For this reason, the tissue penetration of light can reach a few micrometers [13]: in the case of human skin, that corresponds to the stratum corneum. As a consequence, only reflected, scattered light can be considered: there is no light transmitted through a body segment. Advantage: Mid- IR compared to NIR is that the Mid-IR bands produced by glucose, as well as other compounds, are sharper than those of NIR, which are often broad and weak. Limitations: One strong limitation is the poor penetration. Mid-IR is affected by similar problems and confounding factors than NIR, despite glucose bands potentially improved. For instance, some studies have shown significant dependence of skin Mid-IR spectrum on its water content [13]. | ||||
| F. Raman spectroscopy: The Raman spectroscopy is based on the use of a laser light to induce oscillation and rotation in molecules of one solution [2, 5]. The consequent emission of scattered light is influenced by this molecules vibration, which depends on the concentration of the solutes in the solution. Therefore, it is possible to derive an estimation of glucose concentration in human fluids where glucose is present. The Raman spectrum usually considered is in the interval 200–1800 cm- 1 [14, 15]. In this band, Raman spectrum of glucose is quite clearly differentiable from that of other compounds. In fact, Raman spectroscopy usually provides sharper and less overlapped spectra compared for instance to NIR. Advantage: The modest interference from luminescence and fluorescence phenomena [16]. Fixed wavelength lasers at relatively low cost can be used [17]. Recently, an improvement in traditional Raman spectroscopy has been proposed (surface-enhanced Raman spectroscopy), which may increase the sensitivity of the acquisition and or decreasing the acquisition time [18]. Limitations: Main limitations are related to instability of the laser wavelength and intensity, and long spectral acquisition times. Moreover, similarly to other techniques described before, the problem of the interference related to other compounds remains. | ||||
III.CHALLENGES & COUNTERMEASURES IN EFFECTIVE NONINVASIVE GLUCOSE MEASUREMENTS |
||||
| Measurement accuracy and their demonstration largely establish the feasibility of a given technological approach. Selectivity is of paramount importance for a successful noninvasive glucose measurement. | ||||
| (i) The collected spectroscopic information must contain a selective signature for glucose relative to all components within the matrix that can impact the spectroscopic signal. This criterion is true regardless of whether the measurement is based on a direct or indirect approach. The basis of chemical selectivity must be established and clearly distinguished. Analyte distinction is particularly important given the heavy use of multivariate statistical methods of analysis and the propensity to either over model the data or base calibrations on spurious correlations. An emphasis on the net analyte signal and methods to verify analyte specificity from multivariate calibration models is promising. | ||||
| (ii) Instrument performance must be sufficient to enable the collection of the selective glucose signature in a reliable manner relative to background noise. Ultimately, the SNR of the instrumentation defines the limit of detection for glucose and detailed experimental results are needed to establish the level of SNR that is necessary to measure glucose at clinically relevant concentrations. Tissue phantoms provide an excellent means to establish the relationship between the instrumental SNR and the limit of detection. Instrumentation must then be designed to provide this level of performance for spectral data collected noninvasively from living tissue. | ||||
| (iii)The physical and chemical properties of the measurement site greatly influence accuracy of noninvasive clinical measurements. Noteworthy physical parameters include thickness, scattering properties, and temperature of the tissue at the measurement site. Chemical issues center on the molecular makeup of the tissue (water, protein, fats, amino acids, glycolytic structures, etc.) and the heterogeneous distribution of these chemical components throughout the measurement site. | ||||
| (iv)Thickness of the tissue is critical because it determines the number of analyte molecules detected, thereby defining the limit of detection for a given instrumental SNR. Basically, the sampled tissue layer must be sufficiently thick to provide enough analyte molecules within the optical path to generate a signal that can be distinguished from the background noise. If tissue layers are too thick, however, excessive scattering can result in a loss of raw signal and the SNR is reduced. | ||||
| (v) Distribution of the tissue structure within a measurement site must also be understood and its impact on the analytical measurement established. For spectroscopic methods, the incident light enters the tissue and interacts with a multitude of cellular and non cellular structures as it propagates through the tissue. Depending on the measurement site and type of spectroscopy, the measured light might interact with a wide variety of tissue structures. For skin measurements, the light can potentially interact with the epidermis, dermis, and subcutaneous tissue, each of which presents a different chemical composition. If one assumes that the glucose is primarily located in the aqueous fraction of the tissue, then the tissue matrix can be roughly divided into three compartments, the interstitial fluid (outside the cells), intracellular fluid (inside the cells), and blood. Although the concentration of glucose in the interstitial fluid is known to match that in blood under steady-state conditions, the concentration of glucose can vary greatly in these three compartments depending on the mass transport and reaction kinetic properties of different regions within the tissue matrix. Clearly, many factors can influence the concentration of glucose in the localized region of the measurement. | ||||
| (vi)The robustness of the calibration is a critical parameter that must be established before any noninvasive measurement technology will be useful. | ||||
| Important issues include | ||||
| (1) The ability to collect reliable spectra for each measurement; | ||||
| (2) A protocol required to establish a working calibration model; | ||||
| (3) Time stability of a calibration model; and | ||||
| (4) Sensitivity of the calibration model to external factors, such as ambient temperature and vibrations. These issues go far beyond demonstrating the feasibility of a noninvasive spectroscopic sensor but pertain to its eventual practical implementation. [19] | ||||
IV. PRINCIPLE OF MEASUREMENT |
||||
| Electromagnetic waves in the wavelength range between 780 and 2500 nm (equivalent to wave numbers from 12820 to 4000 cm−1) are known as the near-infrared (NIR) wavelength region, and between 2500 and 25000 nm (equivalent to wave numbers from 4000 to 400 cm−1) as mid-infrared (MIR) wavelengths. | ||||
| The concentration of blood glucose refers to the concentration of glucose in blood. The molecular formula of glucose is C6H12O6 and several hydroxyl and methyl groups are contained in this structure. They are main hydrogen functional groups whose absorption occurs in the near infrared region. According to the characteristics of molecular structure, the absorption spectra of glucose and water in the near infrared region are shown in Fig. 1. | ||||
| The second overtone absorption of glucose molecule is in the spectral region between 1100 and 1300 nm and the first overtone absorption of glucose molecule is in the region of 1500 to 1800 nm. This information provides the theoretical basis for the measurement of blood glucose using near infrared spectroscopy. According to the Beer-Lambert law, the relationship between the intensity of incident optical radiation I0, the transmitted intensity I and the path length L of light passing in the analyte is expressed as | ||||
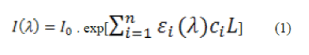 |
||||
| where εi (lamda) and ci are the molar absorptivities and concentrations for each of the n components in the sample, respectively. The transmitted light intensity changes with the concentration of compounds in the matter, thereby, the compound’s concentration can be obtained by the measurement of light transmission through the medium. However, there are a number of substances other than glucose alone in the human body; for example, proteins, water, etc. that also have absorptive characteristics in the NIR region that overlap with the glucose spectrum. Therefore, the measurement of the transmitted light intensity is generally made over multiple wavelengths. On the other hand, the Beer-Lambert law only describes the ideal transmission scenario. In fact, light particles (photons) do not travel along a straight path for the noninvasive glucose measurement. Both the absorption caused by absorbers and the scattering caused by scatters attenuate the incoming light intensity [20]. | ||||
V. PROPOSED METHODOLOGY |
||||
| In recent time various optical based principles have been proposed for noninvasive blood glucose monitoring. NIR & MIR spectroscopy based non invasive techniques for determining the blood glucose concentration had been demonstrated by many research groups and excellent progress has been made in past few years. | ||||
| In Figure 2. The Light emitting diode can be used to measure the NIR or MIR spectra of the human finger tip. A device with LED in the Infra red range can be used for non invasive measurement of blood glucose. The proposed system in figure 2. has been equipped with light emitting diode, finger positioning system & photodiode. An analog to digital convertor is used to convert analog signal to digital signal. Amplifier to strengthen the received feeble bio signal. Micro processer based circuitry converts the values into corresponding blood glucose value, which is then displayed. | ||||
| The probe contains light sources and detectors operating in the near-infrared & mid-infrared spectral region. To provide user expediency and compliance pneumatic cuffs had been used. These cuffs produce over systolic pressure to occlude blood flow. This provides a special adaptive mechanism for easy positioning of wide range of finger sizes with a suitable grip on it. | ||||
| Glucose decreases the mismatch in refractive index between scatterers and their surrounding media, leading to a smaller scattering coefficient and, consequently, a shorter optical path. As a result, with the growing concentration of glucose, fewer photons are absorbed and the light intensity increases. The technology is based on the direct effect of glucose on the scattering properties of the organ. [2]. | ||||
VI.CONCLUSION |
||||
| Continuous non invasive glucose monitoring provides an added advantage such as magnitude, duration and frequency of glucose level fluctuations. This data can prevent complications arise due to hypo and hyper glycemic conditions. Moreover it can activate the alarm system to indicate high or low blood glucose levels. Non invasive blood glucose detecting device can reduce work load of hospital staff, good patient compliance, better diabetic control regimen and less diabetes related mortality rate. | ||||
| This study demonstrated the problems and countermeasures to monitor non invasive blood glucose level. The investigation into non invasive measurement techniques for blood glucose indicates that blood glucose signal is very weak and overlapped by water absorption. Direct absorption measurement is extremely difficult due to these phenomenons. Advent of certain new or hybrid technology can provide optimal solution and requires further investigation. | ||||
Figures at a glance |
||||
|
||||
References |
||||
|