ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
K. Venkateswar Reddy1 ,T. Vijayalakshmi2, A. Uma3, L. saida3 and S. Tirosha4
|
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Release of paper and board mill industry effluents on to the agricultural lands causes indicative changes in nutrient cycling and organic matter processing. In the present study, paper and board mills industry effluent discharged soil (test) and undischarged soil (control) were collected from the surrounding areas of paper and board mills industry. The physicochemical, biological properties and soil enzyme activities such as cellulase, amylase, protease and urease were examined. The experimental results indicated that, most of the physicochemical properties such as sand, silt, clay, electrical conductivity, water holding capacity, organic matter and total nitrogen, phosphorous and potassium, microbial population and selected enzyme activities were significantly higher in the test sample than in the control. Additionally, activities were increased with increasing the incubation period up to 21 d over 0d, however, activities were adversely affected at 28 .Furthermore, relatively higher activities were observed in soil incubated in the presence of substrate than in the absence of substrate.
Keywords |
| paper and board mills, industry effluent, physicochemical, biological, soil enzymes. |
INTRODUCTION |
| Soil is an important system of terrestrial ecosystem. There is a direct impact of pollutants on minerals, organic matter and microbial community of soil (1). The discharge of industrial effluents especially without treatment may have profound influence on physico-chemical and biological properties of soil related to soil fertility. A wealth of information on occurrence of changes in properties of soils due to discharge of effluents from other industries is available such as cotton ginning mill (2), sugar industry (3), paper industry (4), dairy industry (5), and dairy wastewater Thus, determination of enzyme activity and microbial biomass, soil physico-chemical parameters seems to be the best approach for evaluating the state of microbial activity. (6) The pulp and paper industry is one of the major consumers of water. Pulp and paper industrial wastewaters usually contain halogenated organic materials, because general use is made of chlorine containing compounds as bleaching agents during pulp and paper manufacture. . Chlorination is generally the first stage in Kraft pulping and during this treatment phase chlorinated organic compounds are produced. Several methods have been attempted for decolourization and detoxification of bleached Kraft effluents. These include physicochemical and biotechnological methods. The problems underlying the physicochemical treatments are those associated with cost and reliability. Biotechnological methods have the potential to eliminate/reduce the problems associated with physicochemical methods.(7).In the present study, an attempt has, therefore been made to find out the impact of effluents of paper and board mills industry on soil physical (pH,EC, water holding capacity), chemical (organic matter, total nitrogen, phosphorus and potassium), biological (bacterial and fungal populations) properties and selected soil enzyme activities. |
MATERIALS & METHODS |
| A.Collection of soil samples |
| Soil samples were collected from the surrounding areas (1/4 km) of paper and board mills industrial area, patancheru, madak district of Andhra Pradesh, India. Soil sample without effluent discharges served as control was collected from adjacent site (1 km away) of industry. Soil samples both with and without effluents were used for determination of physico-chemical, biological and enzyme activities. These two soil samples were air dried and mixed thoroughly to increase homogeneity and shifted to <2 mm sieves for determination of soil texture. |
| B. Physico-chemical and biological properties of soil |
| The physico-chemical and biological properties of test and control soils were determined by the following standard procedures. The soil particles like sand, silt and clay contents were analyzed with the use of different sieves by the method of Alexander(8). Whereas water holding capacity, organic carbon, total nitrogen, and soluble phosphorous of soil samples were determined by the methods of a Johnson & Ulrich(9), Walkey-black (10), Microkjeldhal (11) and Kurrevich and Shcherbakova (12), respectively. Electric conductivity and pH were determined by Elicoconductivitymeter and pH meters, respectively. |
| C. Biological parameters |
| Micro flora such as bacterial and fungal populations of both soil samples were enumerated by serial dilution technique. One gram of each soil sample was serially diluted and 0.1 ml was spread with a sterile spreader on nutrient agar medium and Czapeck-Dox agar medium for the isolation of bacteria and fungi, respectively. Nutrient agar plates were incubated at 37 ºC for 24 h, where as Czapeck-Dox plates were at room temperature for 7d. After incubation period, colonies formed on the surface of the medium were counted by colony counter (2). |
| D. Enzyme assays |
| Five grams of soil samples contaminated with/without effluents of paper and board mills were transferred to test tubes. Soil samples were maintained at 60% water holding capacity at room temperature in the laboratory(28 ± 4 °C).Triplicate soil samples of each waste water treated and controls were withdrawn at periodic intervals to determine the soil enzyme activities as detailed earlier by Tu (13). The method employed for the assay of amylase, protease,cellulase and urease were essentially the same developed by Cole (14), Speir and Ross (15), Pancholy and Rice (16), and Zantua and Bremner (17), respectively. The soil samples were transferred to 250mL of Erlenmeyer flasks and one mL of toluene was added. After 15min, 6mL of 0.2 M acetate phosphate buffer (pH 5.5 ) containing either 2% starch(amylase), 2% casein (protease) and 1% CMC (cellulase) were added to soil samples and flasks were plugged with cotton and held for 48 h (amylase), 24 h (protease), and 30min (cellulase) at 30 °C. After incubation, soil extracts were passed through whatmann filter paper, then glucose (amylase,cellulase) and tyrosine (protease) contents in the filtrate were determined by the methods of Nelson-Somagyi(18), Lowry(19), respectively. For urease the method comprises release of ammonia up on incubation of soil with 4mL of sodium phosphate buffer (pH 7.0), 1mL of 1 M urea solution incubated for 30 min and 10ml of 2M KCl was added and kept at 4 ºC for 15 min and centrifuged, then 0.5mL of Nesslers reagent followed by 3.5mL of distilled water were added and the color was read at 495nm, in an digital spectrophotometer. |
RESULTS AND DISCUSSION |
| Soil samples of both with and without effluents discharge were analysed for their physico chemical properties and their results were represented in table 1. Soil samples with paper and board mills industry effluent underwent changes in all measured parameters of physical and chemical properties in comparison to control. There was no noticeable change in the pH of the test soil over control. However, soil texture in terms of percentage of clay, silt, sand were 34,32,34 in test and 33,32,35 in the control soils, respectively. Higher water holding capacity was observed in test soil than control, values were found to be 2.5 and 1.3 mL g−1, respectively. The electrical conductivity of both test and control soils were 275 and 151 μMhos cm−1, respectively. Increased water holding capacity and electrical conductivity in contaminated soil may be due to the accumulation of organic waste such as amino acid residues, acids and alkalis in the paper and board mills industry effluents. |
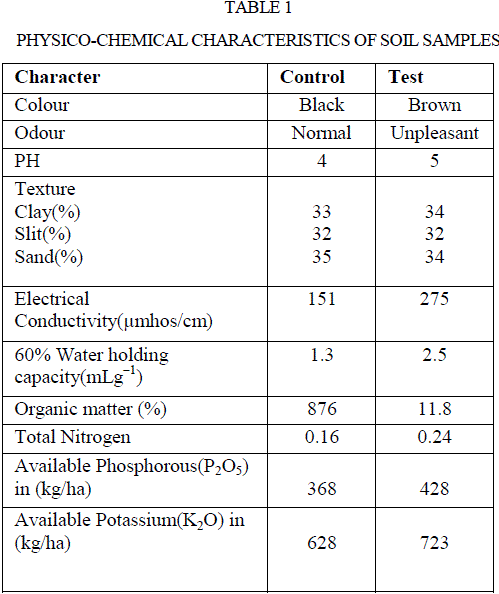 |
| The results were in conformity with the studies of Sparling et al (20), Narasimha et al (2), Poonkothai and Parvatham (21), and Xiao et al (22) had increased electrical conductivity in soil contaminated by the effluents of dairy, cotton ginning, automobile, and black liquor for straw pulping industries, respectively. The parameters like organic matter percentage, total nitrogen, phosphorus, potassium were higher in test soil than the control soil. The values of above properties of test sample were 11.8 %, 0.24 gkg−1, 428 kg ha−1, 723 kg ha−1, and control soils were 8.76 %, 0.16 g kg−1, 368 kg ha−1, 628 kg ha−1, respectively (Table 1). Higher organic matter of the polluted soil may be due to the discharge of waste water in organic nature. Also, increased organic matter enhanced soil enzyme activity. Narasimha et al (2) and Kaushik et al (23) made similar reports on the discharge of effluents from cotton ginning and distillery industries, respectively. Thus, soil is a potent system of terrestrial ecosystem, and direct discharge of industrial effluents especially that without treatment may have profound influence on physico-chemical and biological properties of soil related to soil fertility (24). Similarly, discharge of effluents from various industries like sugar industry (1), dairy factory (5) and petrochemical industry (25) influenced the physico-chemical properties of soil. This is due to organic waste that may contribute to maintain or increase the organic matter and nutrient content in the soil (26). |
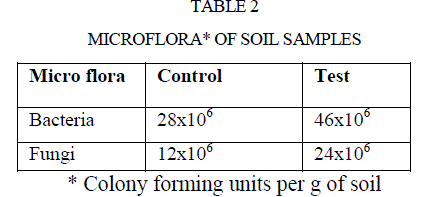
| The microorganisms play a vital role in nutrient cycling and soil fertility. Bacteria and fungi synthesize and secrete enzymes such as amylase, cellulose, ureases, proteases, phosphates, pectinase are extracellular. Those microbial secreted enzymes constitute an important part of soil matrix as extra cellular enzymes (7). Thus, there is a considerable interest in the study of enzyme activities of soil (27), because such activities may reflect the potential capacity of a soil to form certain biological transformation of importance to soil fertility (28). Micro flora of both samples were enumerated and listed in table 2. |
| Polluted soil caused two fold increases in bacterial and fungal population compared to control soil(Table 2). |
 |
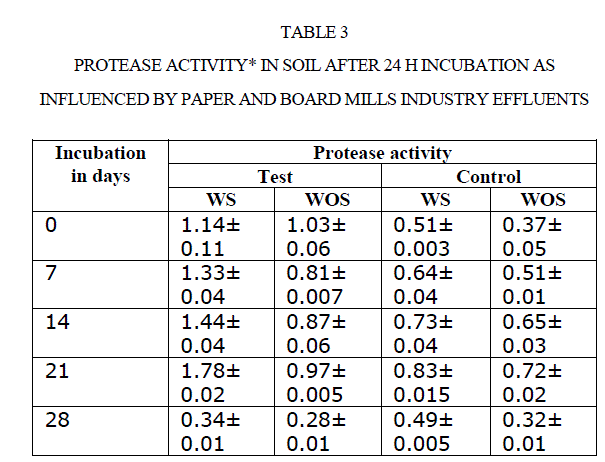 |
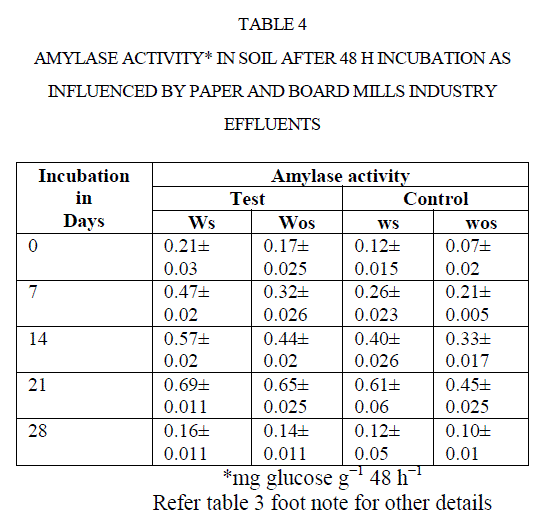
| There was an increase in activity up to 21 d incubation, there after activities were adversely affected. For instance, amylase activity in polluted soil increased from 0.21to 0.69 mg glucose equivalents g−1 48 h−1 from 0 to 21 d. Later it was decreased to 0.16 mg GE g−1 48 h−1 at 28 d (Table 4). Comparison of soil amylase activity in soil samples with/without effluents discharged revealed that the soil polluted with effluents stimulated the amylase activity by ~2 fold than control soil. Narasimha et al(2)made a similar observation in soils polluted with cotton ginning mill effluents stimulated the soil Amylase activity. |
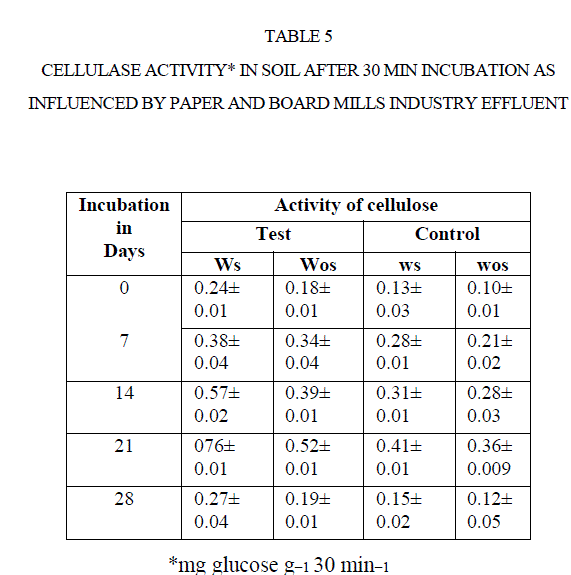
| The cellulase activity was measured in terms of release of glucose from CMC. There was an increase the formation of glucose with increasing soil incubation such as0, 7, 14, and 21 d. The cellulase activity decreased after21 d of incubations (Table 5) ] |
 |
| For instance, the cellulase activity in test soil increased from 0.24 mg GE g−1 30 min−1 to 0.76 mg GE g−1 30 min−1 0to 21 d. Later it was decreased to 0.27 mg GE g−1 30 min−1 at 28 d incubation. Same was reported by Narasimha et al (2)in soils polluted with effluents of cotton ginning mills stimulated the soil cellulase activity at early d of incubation. |
| Urea is an organic chemical complex used mainly as nitrogenous fertilizer in agriculture. Conversion of this nitrogen to inorganic nitrogen-ammonia and carbon dioxide takes place due to activity of urease enzyme, secreted by certain microorganisms and is responsible for supply of nitrogenous demand to growing crops. Assay of urease activity in soil samples involves quantification of ammonia released upon hydrolysis of urea. Urease activity in soils with/without effluents discharges was measured and results were shown in table 6. Urease activity also increased up to 21d of incubation and later declined. For instance the urease activity in test soil with substrate at 0 d was 0.40 mg NH4+ - N g−1 30 min−1, it was increased to 0.70mg NH4+-N g−1 30 min−1 at 21 d and declined to 0.26 mg NH4+- N g−1 30 min−1 at 28 d (Table 6). |
 |
| Similar trend was also noticed in other samples also. Similar results were noticed by Narasimha et al (24) that urea’s activity was increased in the first week of incubation, there after declined in soil contaminated with cotton ginning mill effluents. |
CONCLUSIONS |
| The present study clearly indicates that the disposal of effluents from paper and board mills industry alters the physico-chemical; biological properties and activities of enzymes such as protease, amylase, cellulase and urease were stimulated in soil over control. Nonetheless, prolonged incubation causes adverse effects. Thus, this observation, therefore, greatly warrants a prior treatment of paper and board mills industry effluents before discharging into a water body or onto agricultural land and additional research will be necessary to discriminate the type of these extra cellular enzyme producing microorganisms (genera and species). |
ACKNOWLEDGEMENT |
| Authors are thankful to the staff paper and board mills industry, patancheru, madak dist A.P., India for their timely help and encouraging us throughout this study. |
References |
|