ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
R.Ratheesh1 and K.Viswanathan2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Polyaniline doped with PTSA in three different concentrations are prepared by chemical synthesis method and their thermal conductivity and electrical conductivity are measured. It is seen that the electrical conductivity increases linearly with temperature, showing the semiconducting nature of the sample. Also, the conductivity is maximum for highest doping concentration. The electrical conductivity is found to increase with increase of temperature, suggesting its thermal stability. The elemental analysis is carried out in order to understand the composition of the polymer with various elements such as C, H, N and S present in the doped samples. The higher value of %S in the sample having maximum level of doping(y=0.5) and conductivity, shows the effective doping of polymer chain. Also the %S decreases with decrease in the doping level (y=0.3 and y=0.1) which shows conductivity lower than that having maximum level of doping. The energy gap decreases with increase in doping level and thereby conductivity increases. Mott’s variable range hopping method is used to explain the conductivity behaviour. A detailed study has been made on the electron transport properties of PTSA doped polyaniline in the temperature range of 300 - 400 K. It is seen that there is more than one mechanism involved in charge transfer in polyaniline depending on the temperature range. Charge transfer in PANI is peculiar, so that no one mechanism can completely describe it. However, to some extent, conduction in polyaniline can be explained in terms of Mott’s three dimensional variable range hopping model, Kivelson model and Arrhenius model.
Keywords |
| Poly Aniline, polymerization, emeraldine, conductivity, Variable range hopping, Kivelson model, Arrhenius model. |
INTRODUCTION |
| In recent years, conducting polymers has received worldwide attention due to their electronic and electrical properties. These polymers have large number of applications ranging from energy storage device, gas sensors, anticorrosive materials, electromagnetic interference shielding, and electrostatic charge dissipation, organic light emitting diodes, active electrode materials in batteries, plastic solar cells and supporting material for catalysis [1-10]. |
| A large number of studies on conductivity of doped polyaniline have been performed during the last decade to understand the charge transport mechanism [11-15,21].Among all the conducting polymers, conducting polyaniline is of great attention due to the fact that, unlike inorganic metals and semiconductors, both the synthesis and chemical modification of organic materials offer unlimited possibilities. Properties such as good environmental stability, solubility and simple non-redox doping by protonic acids have made doped polyaniline (PANI) the most suitable material for studies of electron transport mechanism [16-21].All most all studies of conducting polymers are still confined to the transport studies of heavily protonated polyanilines only. In this work, emphasis was given on making an extensive study on the transport properties at low temperature in three different level protonated polyaniline. In the present study, we report the chemical synthesis of aniline doped with p-toluene sulfonic acid (PTSA) and its characterization by electrical conductivity measurements. |
II. EXPERIMENTAL |
| Aniline (monomer), Ammonium persulphate (oxidant) para- toluenesulphonic acid (dopant, PTSA), Hydrochloric acid, m-cresol Methanol and Acetone were obtained from Merck. All the chemicals purchased were used as they received. |
Synthesis of emeraldine salt |
| 9 ml aniline (0.1mol) was taken in a round bottom flask containing 150 ml of distilled water and 10 ml concentrated hydrochloric acid. Then 11g (0.05M) of ammonium persulphate was dissolved in 100 ml of distilled water, and then added drop-wise to a stirred solution of 0.1 mol of aniline dissolved in hydrochloric acid, pre cooled to 0-50C. Ammonium peroxy disulphate is added very slowly with constant and stirring is continued for 2 hours to ensure completion of the reaction. |
| The precipitated emeraldine salt is filtered and washed with distilled water until the filtrate is colourless. The precipitate is then washed with methanol to remove oligomeric impurities. Finally it is washed with acetone, to remove water content, and kept overnight for filtering. The precipitate is collected in a beaker and dried under dynamic vacuum at 700C for 6 hours. The dried precipitate is then crusted into fine powder to get emeraldine salt. |
Preparation of emeraldine base |
| The prepared emeraldine salt (powder form) was taken in a round bottom flask containing 20 ml ammonia solution and 80 ml of water is stirred continuously for 24 hours. The precipitate (blue emeraldine base) is filtered and washed with distilled water, methanol and finally with acetone. After keeping over night for filtering, the precipitates are collected and dried under dynamic vacuum at 70oC for 6 hours. The dried precipitate is then crusted in to fine powder to get emeraldine base. |
Emeraldine base doped with para-toluene sulphonic acid (PTSA) |
| In the present study, para-toluene sulphonic acid (PTSA) was used as a dopant. The PANI – (PTSA)y was prepared in three different doping levels [The doping level is defined as Y= (mole acid)/ (mole PANI)]. The PANI – (PTSA)Y was prepared by a thorough mixing of emeraldine base PANI with para-toluene sulponic acid (PTSA) using a glass mortar and pestle to obtain required doping levels, known as the molar ratio of PTSA to phenyl – nitrogen repeat units (S/N). |
| 1. The PANI – (PTSA)0.5 was prepared by a thorough mixing of 0. 012 M emeraldine base PANI with 0.006 M paratoluene sulphonic acid (PTSA) using a glass mortar and pestle to obtain the required doping level Y= 0.5. This is the maximum level of doping. |
| 2. The PANI – (PTSA)0.3 was prepared by a thorough mixing of 0.012 M emeraldine base PANI with 0.0036 M paratoluene sulphonic acid (PTSA) using a glass mortar and pestle to obtain the required doping level y= 0.3. |
| 3. The PANI – (PTSA)0.1 was prepared by a thorough mixing of 0.012 M emeraldine base PANI with 0.0012M paratoluene sulphonic acid (PTSA) using a glass mortar and pestle to obtain the required doping level Y=0.1. |
| For all studies, the doped PANI with Y =0.5, Y= 0.3 and Y= 0.1 are used. |
III. MEASUREMENTS |
| The prepared emeraldine salt powder was made into a pellet for conductivity studies using four probe apparatus. Electrical conductivity was calculated using the following equation [10] |
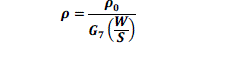 |
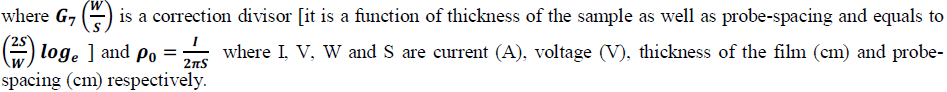 |
| The conductivity was measured at different temperatures (from 300 K to 400 K). The current (I) was kept constant during the experiment. |
IV. RESULTS AND DISCUSSION |
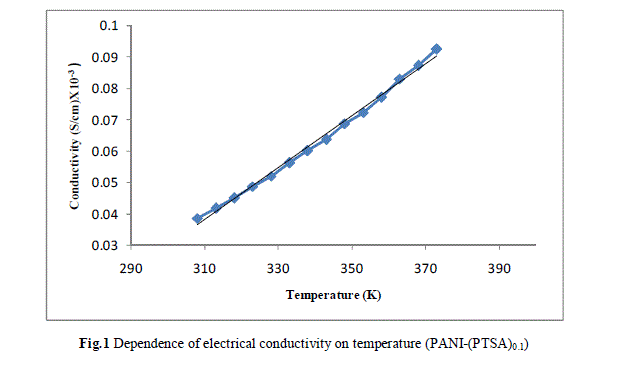
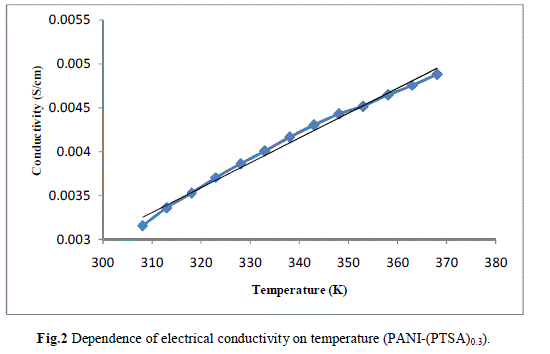
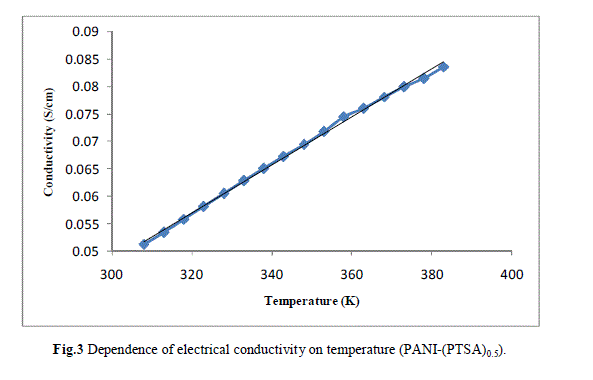
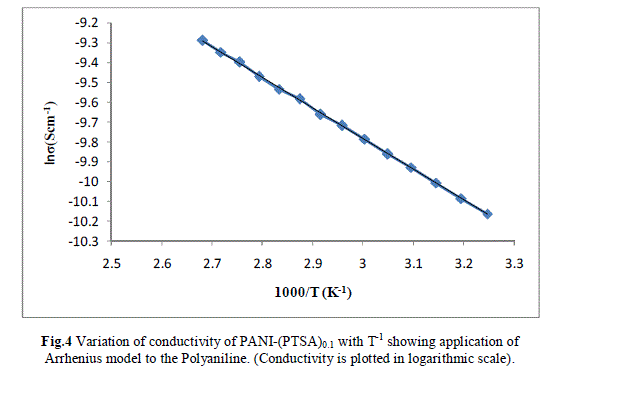
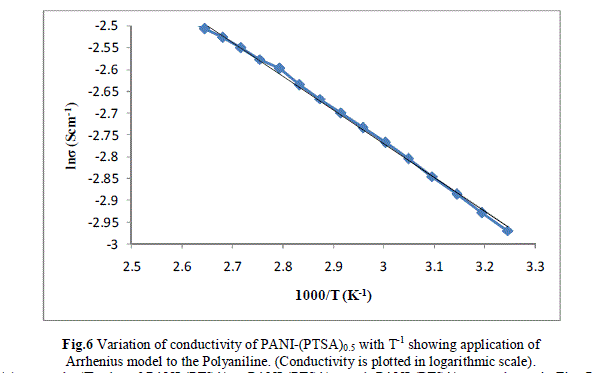
| Measurement of the d.c conductivity of PTSA doped polyaniline samples have been made in the temperature range 300 K-400 K using four probe apparatus.The change in conductivity with increasing temperature of PANI-(PTSA)0.1, PANI-(PTSA)0.3 and PANI-(PTSA)0.5 are shown in Fig.1, 2 and 3 respectively. It is observed from the Figs. 1, 2 and 3 that the conductivity of para-toluene sulphonic acid (PTSA) doped polyaniline (PANI) at different doping levels (Y = 0.1, Y =0.3, Y =0.5) behaves like a semiconductor and its electrical conductivity increases with increase in temperature. On comparison with the standard values of electrical conductivity for traditional semiconductors, it is observed that the initial electrical conductivity of the PTSA doped PANI is well within the semiconductor region. The initial electrical conductivity was observed around 10-2 S cm-1 for PANI-(PTSA)0.5, 10-3 S cm-1 for PANI-(PTSA)0.3 and 10-5 S cm-1 for PANI-(PTSA)0.1 respectively at 35°C. However, the temperature dependence of electrical conductivity of PTSA doped PANI (in all the three doping levels) increases with increase of temperature, which suggests its thermal stability. The temperature dependence of resistivity was fitted to Arrhenius type equation and measured values were plotted logarithmically as a function of reciprocal of temperature. The Figs. 4, 5 and 6 shows the variation of In σ with inverse temperature of PANI-(PTSA)0.1, PANI-(PTSA)0.3 and PANI-(PTSA)0.5 respectively. |
 |
 |
 |
 |
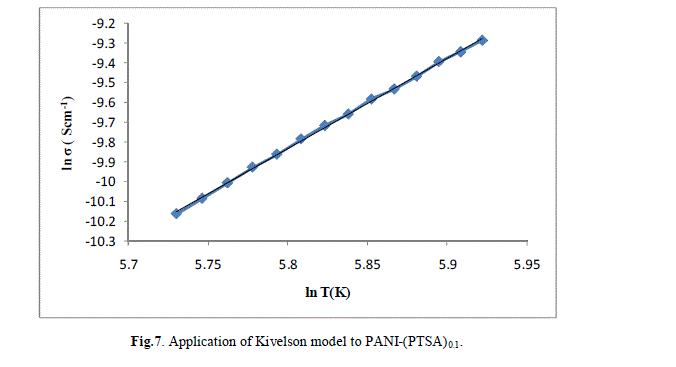
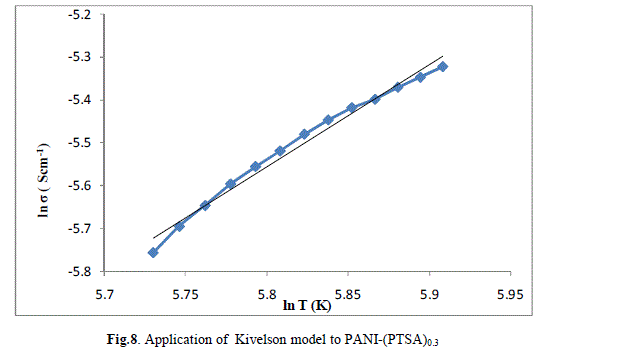
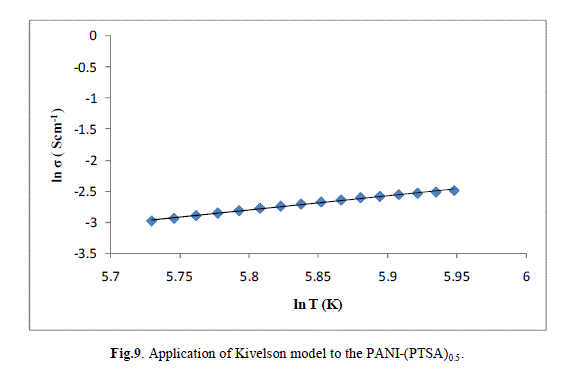
| The ln (σ) verses ln (T) plot of PANI-(PTSA)0.1, PANI-(PTSA)0.3 and PANI-(PTSA)0.5 are shown in Figs.7, 8 and 9 respectively. The ln (σ) verses ln (T) plot shows that the power law behaviour or Kivelson model, σdc = ATn , fits to the experimental data. |
 |
 |
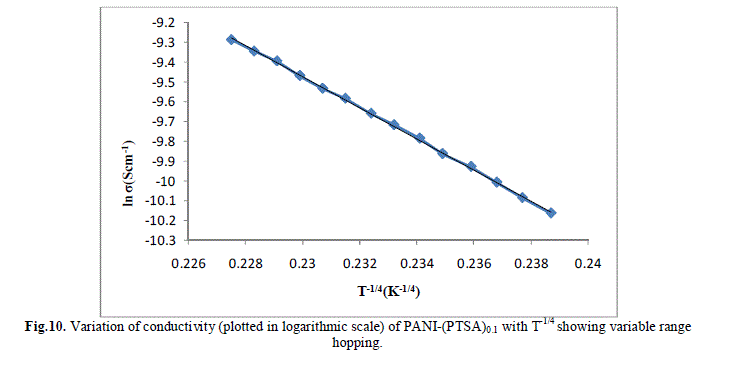
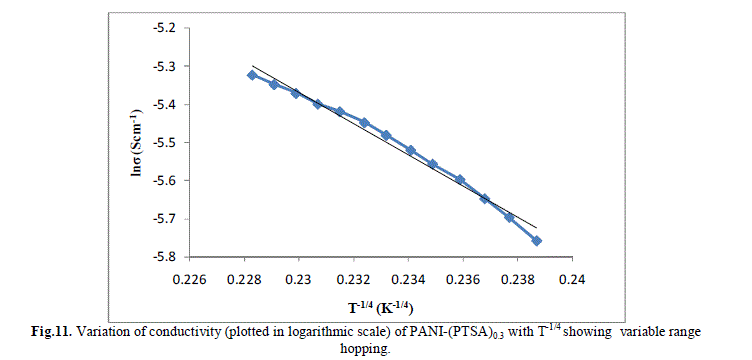
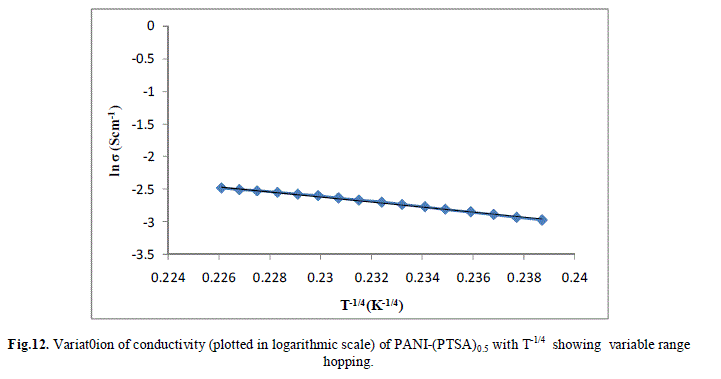
| Several models have been used to explain the conductivity behaviour in the polymers. A mechanism proposed to explain the DC-conductivity in disordered and amorphous material is Mott’s variable range hopping. This mechanism is based on the idea that carriers tend to hope larger distances to sites which lies energetically closer rather than to their nearest neighbours. According to VRH model, the temperature dependent of conductivity follows the formula |
 |
 |
| The band gap and slope calculated from the graph are shown in Table 1 along with the conductivity of the samples. These are shown in Fig 13.a, b & c. It is clear that the energy gap decreases with increase in doping level and thereby conductivity increases. |
 |
 |
| The elemental analysis was carried out in order to know about the composition of the polymer with various elements such as C, H, N and S present in the doped samples. Table 2 shows the values for the typical emeraldine salt phase of the polymer. These are shown in Fig. 14. The higher value of %S in polyaniline confirms the efficient doping of the polymer chains especially iminic nitrogen atom which is further supported by conductivity measurements discussed. In the three doped samples, the higher value of %S is present in the sample having maximum level of doping (y=0.5) and conductivity, shows the effective doping of polymer chain. Also the %S decreases with decrease in the doping level (y=0.3 and y=0.1) which shows conductivity lower than that having maximum level of doping. |
 |
 |
V. CONCLUSIONS |
| Polyaniline was prepared by chemical synthesis method and doped with Para Toluene Sulphonic acid at three different molar ratios. Thermal conductivity and electrical conductivity of the sample were measured. From the temperature vs conductivity plots for the three samples, it is seen that the electrical conductivity increases linearly with temperature, showing the semiconducting nature of the sample. Also, the conductivity is maximum for maximum doping concentration. The initial electrical conductivity was observed around 10-2 S cm-1 for PANI- (PTSA)0.5, 10-3 S cm-1 for PANI- (PTSA)0.3 and 10-5 S cm-1 for PANI- (PTSA)0.1 respectively at room temperature. However, the temperature dependence of electrical conductivity of PTSA doped PANI (in all the three doping levels) increases with increase of temperature, which suggests its thermal stability. The temperature dependence of resistivity was fitted to Arrhenius type equation and measured values were plotted logarithmically as a function of reciprocal of temperature. |
| The elemental analysis was carried out in order to know about the composition of the polymer with various elements such as C, H, N and S present in the doped samples. In the three doped samples, the higher value of %S is present in the sample having maximum level of doping(y=0.5) and conductivity, shows the effective doping of polymer chain. Also the %S decreases with decrease in the doping level (y=0.3 and y=0.1) which shows conductivity lower than that having maximum level of doping. The band gap and slope for different PANI-PTSA calculated from the graph. From the calculation it is clear that the energy gap decreases with increase in doping level and thereby conductivity increases. Several models have been used to explain the conductivity behaviour in the polymers. A mechanism proposed to explain the dc conductivity in disordered and amorphous material is Mott’s variable range hopping. This mechanism is based on the idea that in conducting polymers, charge carriers tend to hope larger distances to sites which lies energetically closer rather than to their nearest neighbours. It seems that the graph shows best fit to the experimental data. The ln (σ) verses ln (T) plot shows that the power law behaviour or Kivelson model, σdc = ATn, fits to the experimental data. |
| Polyaniline doped with p-toluene sulfonic acid was synthesized by chemical polymerization method using Ammonium peroxydisulfate as an oxidant. Detailed study has been made on the electron transport properties of PTSA doped polyaniline in the temperature range of 300 - 400 K. The conductivity of the prepared samples shows an increasing trend with increasing temperature. It is clear that, there is more than one mechanism involved in charge transfer in polyaniline depending on the temperature range. Charge transfer in PANI is peculiar, so that no one mechanism can completely describe it. However, to some extent, conduction in polyaniline can be explained in terms of Mott’s three dimensional variable range hopping model Kivelson model and Arrhenius model. |
References |
|