e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
1Department of Pharmaceutics, RR College of Pharmacy, Bangalore 560090, Karnataka, India.
2Sri Venkateswara College of Pharmacy, Chittoor 517127, Andhra Pradesh, India.
3Department of Chemistry, JNTUA Anantapur 515001, Andhra Pradesh, India.
4Department of Pharmaceutics, Gautham College of Pharmacy, Bangalore 560032, Karnataka, India.
Received: 26/08/2014 Accepted: 17/09/2014
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
The unidirectional release buccal patches of carbamazepine (CBZ) were designed in total of 36 formulations using a 32 full factorial design. Different hydrophilic polymers were used in the formulation along with chitosan-HPMC matrix. The thickness of the prepared patches varied between 0.3 ± 0.10 and 0.5 ± 0.15 mm. The mass of the patches varied from 142.4 ± 0.22 and 160.2 ± 0.13 mg. The pH of the prepared patches was in the range of 4.9±0.01 to 6.2±0.06. The drug loading efficiency of the patches varied between 18.1±0.5 and 19.8±0.1 and showed folding endurance of ˃188. Out of these 36 formulations, five formulations (FA42, FA46, FB38, FB49 and FB52) showed high folding endurance of ˃255. These patches were selected for further evaluation such as swelling studies, ex vivo mucoadhesion time, ex vivo mucoadhesive strength, in vitro drug release, ex vivo permeation, accelerated stability studies, DSC, FTIR and X-ray diffraction spectral studies. The percentage of swelling was higher up to 53 ± 1.1 for FB49 after 160 min. The percentage swelling was increased in the following order, FA42 < FB38 < FA46 < FB52 < FB49. The ex vivo mucoadhesion time of selected buccal patches was in the range of 112 ± 3.9 to 167 ± 3.1 min. The highest mucoadhesive force was observed with formulation FA42. In vitro release revealed that formulation FB49 showed maximum release of 99.9% after 90 min and followed by FB52 (105 min), FA46 (135 min), FB38 and FA42 (150 min). CBZ permeation through the porcine buccal mucosa was investigated by using Franz diffusion cell. There was a good correlation between in vitro drug release and ex vivo permeation studies. The accelerated stability study of tested patches showed no significant change in drug content, mucoadhesion time and surface pH, observed in the beginning of the study.
Carbamazipine, mucoadhesion, unidirectional, porcine buccal mucosa
Transmucosal routes of drug delivery provide distinct advantages over peroral administration for systemic drug delivery. Buccal route of drug delivery provides the direct access to the systemic circulation through the jugular vein bypassing the first pass hepatic metabolism leading to high bioavailability. Buccal route has other advantages such as excellent accessibility, very low enzymatic activity, easy withdrawal, facility to include permeation enhancer in the formulation and versatility in designing as multidirectional or unidirectional release system for local or systemic action [1]. CBZ is a widely used drug of choice for treatment of psychomotor epilepsy and trigeminal neuralgia. The usual oral administration leads to slow and irregular absorption in the gastro intestinal tract due to poor water solubility of CBZ (170 mg/litre at 24ºC). The plasma half-life ranges from 18 to 60 h following a single oral dose and from 10 to 35 h during chronic therapy, which results poor bioavailability after oral administration [2]. CBZ is characterized by hepatic first-pass effect due to the enzymatic auto-induction of its metabolism. The biotransformation of CBZ into CBZ 10,11epoxide may initiate adverse drug reactions like dizziness, diplopia and light headedness. This metabolic conversion can be overcome by bypassing hepatic first pass metabolism. This metabolite associated adverse effects besides the need of a prompt action to make CBZ a potential candidate for the development of mucoadhesive buccal patches [3].
The gift sample of CBZ was received from Caplin point Pharma Ltd, Puducherry, India. The mucoadhesive polymers Chitosan, Carbopol 934, Hydroxypropyl methyl cellulose and Poly vinyl alcohol were purchased from Sigma Chemicals, Bangalore, India. Plasticizers and other chemicals like propylene glycol, polyethylene glycol 400 and aspartame were purchased from SD Fine Chem Ltd, Bangalore, India. Biaxially-oriented polypropylene (BOPP) film was supplied by Pidilite®, India. Simulated saliva was prepared using a buffer solution (pH 6.8) containing Sodium chloride (2.34 g), Calcium chloride dihydrate (0.167 g), Potassium dihydrogen phosphate (1.632 g) and 0.2 M sodium hydroxide to give pH 6.2 in 1 litre of distilled water.
The buccal patches of CBZ were prepared by solvent casting technique using a Teflon coated Petri dishes. Initially the formulations were designed by 32 full factorial designs (IBM® SPSS Statistics Version 20). Two different combinations of polymers CH/HPMC/PVA and CH/HPMC/CP were used in the formulations (Table 1).
The 2% (m/V) of chitosan was dissolved in 10 mL of 4% (m/V) citric acid under occasional stirring for 12 hr. The resulting viscous chitosan solution was filtered through gauze to remove insoluble debris and suspended particles. The aqueous dispersions of polymers were prepared by mixing 2 % w/v solution of CH and HPMC and 1 % w/v solution of PVA or CP in distilled water. The mixtures of polymers (30 ml) in different proportions were homogenized with 5 ml of CBZ dissolved in ethanol (equivalent to 20 mg per 3 cm patch) and 2 ml of Plasticizer (PG/PEG 400). The Final dispersion was homogenized for 2 h and then the air bubble free clear solution was casted on the surface of specially fabricated Teflon coated Petri dish (9.2 cm diameter). Inverted funnel was placed over the Petri dish to avoid sudden evaporation. The Patches were dried at room temperature for 5 h in a horizontal surface and further dried in a hot air oven at 50 °C for 48 h. The dried patches were carefully removed and examined for imperfections and cut into 3 cm diameter patches (equivalent to 20 mg of CBZ).The patches were converted into unidirectional release by pasting on one side with a water impermeable backing layer (Pidilite® BOPP film). The patches were covered in an aluminium foil and preserved in a desiccator at controlled temperature for further studies [4].
Buccal patches (3cm) without BOPP films were selected randomly from each formulation and their thickness were measured individually using digital vernier caliper. The average of three determinations was reported.
Mass uniformity of prepared buccal patches was measured by weighing individually of three randomly selected patches (without BOPP films) from each formulation using an electronic balance. The results were articulated as the average of three determinations.
Folding endurance was measured without backing membrane by folding a patch at the same place for several times till it broke or develop visible cracks or withstand folding up to 250 times without breaking. The average of three determinations was reported [4,5].
Three patches were dissolved separately in 100 mL simulated saliva (pH 6.2) and alcohol mixture (80:20), then filtered through cellulose acetate membrane (0.45 μm). The amount of drug was determined spectrophotometrically at λ max 285 nm (Shimadzu1800, Japan). The averages of results were determined [6].
The surface pH of the patches was determined in order to investigate the possible irritation at the site of application due to pH of patches. The method adopted by Bottenberg et al was used to determine the surface pH. A combined glass electrode was used for this purpose. Each film was allowed to swell by keeping it in contact with 1 ml of deionised water (pH 6.5) for 2 h at room temperature, and the pH was noted by glass electrode brought into contact with the surface of the film and by allowing it to equilibrate for 1 minute. The experiment was performed in triplicate, and average values were reported [4].
The three films were tested for each formulation. After determination of the initial weight of patch without BOPP backing membrane (W0), the sample was allowed to swell on the surface of a 2 % w/V agar plate prepared in simulated saliva pH 6.2. Then it was maintained in an incubator at 37 °C. Weight of the swollen patch (Wt) was measured (n=3) at predetermined time intervals up to 160 min. The percentage of swelling (%S) was calculated using the following equation [4]:
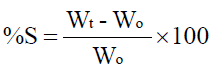
Where Wt is the weight of the swollen patch after time t,
Wo is the initial weight of patch at t=0
The ex vivo mucoadhesion time was determined using USP disintegration apparatus. The beaker was filled with 800 mL of Simulated saliva pH 6.2 maintained at 37±1°C. The segments of porcine buccal mucosa, each of 3 cm length, were glued to the surface of a glass slab, which was then vertically attached to the shaft of apparatus. The buccal patch (3 cm diameter) was hydrated on one surface using simulated saliva pH 6.2 and the hydrated surface was brought into contact with the mucosal membrane. The glass slab was vertically fixed to the apparatus and allowed to move up and down at the rate of 25 cycles per minute. The patch was completely immersed in the buffer solution at the lowest point and was completely out at the highest point. The time required for complete erosion or detachment of the film from the mucosal surface was recorded (mean of three determinations) [8].
The porcine buccal mucosa was carefully excised and separated from connective and adipose tissue and washed with saline. Mucoadhesive strength of the patch was measured on a modified physical balance assembly. The fresh porcine buccal mucosa was cut in to 3 cm length pieces and washed with simulated saliva pH 6.2. A 3 cm2 porcine buccal mucosa was glued on the surface of a dry glass Petri dish. The mucosal surface was placed facing outward. The Petri dish was moistened with 2 mL of simulated saliva pH 6.2. The right side pan of the balance was replaced by a horizontally hanging glass disc fixed with a buccal patch of 3 cm diameter at the down side. Two pans of the balance were balanced with sufficient weight on the left side pan. A weight of 5 gm (w1) was removed from the left side pan, which lowered the pan along with the patch over the mucosa. The balance was kept in contact in this position for 5 min. Then the weight of left pan was gradually increased until the mucoadhesion breaks (w2). The difference in weight (w2 - w1) was measured as mucoadhesive strength. By applying this value in the following equation, mucoadhesive force (kg.m.s-2) was calculated [5]:
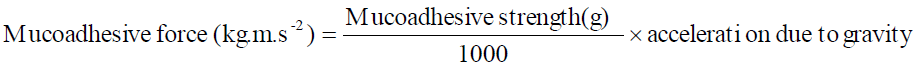
Here, acceleration due to gravity 9.8 ms-2 .
The USP XXIII type 2 rotating paddle dissolution apparatus (Electrolab, EDT-08Lx) was used to study the drug release from buccal patch. The dissolution media consisted of 100 ml of ethanol and simulated saliva solution (pH 6.2) mixture (20:80). The In vitro drug release study was performed at 37±1°C, with a rotation speed of 50 rpm. The one side of buccal patch was attached to the glass disc with cyanoacrylate adhesive. The disc was placed at the bottom of the dissolution jar by facing buccal patch upside. Samples (2mL) were withdrawn at predetermined time intervals and replaced with prewarmed fresh medium. The samples were filtered through 0.45 μm filter and assayed UV- spectrophotometrically at 285 nm. The averages of three determinations were reported [9].
The mechanism of In vitro drug release was determined by fitting the release data to the various kinetic equations such as zeroorder, first-order, Higuchi, and Korsmeyer-Peppas and finding the R2 values of the release profile corresponding to each model.
The ex vivo buccal permeation study of CBZ through the porcine buccal mucosa was performed using a modified Franz type glass diffusion cell at 37±1°C. Porcine buccal mucosa was obtained from a local slaughterhouse and used within 2 h of slaughter. The buccal mucosa was mounted between the donor and receptor compartments by placing outer smooth surface of mucosa towards donor compartment. The buccal patch was placed on the mucosa and the compartments were clamped together. The donor compartment was filled with 1 ml of simulated saliva (pH 6.2). The receptor compartment (200 ml capacity) was filled with phosphate buffer (pH 7.4) and alcohol mixture (80:20) as permeation medium to avoid saturation of CBZ in the medium, and the hydrodynamics in the receptor compartment were maintained by stirring with a magnetic bead at 50 rpm. At predetermined time intervals, a 1ml sample was withdrawn and analyzed. The experiments were performed in triplicate, and average values were reported [10].
Patches were placed in a glass vial wrapped with aluminium foil and kept in a humidity chamber maintained at 40± 0.5°C and 75±5% RH for 6 month. Changes in the appearance and drug content, surface pH and ex vivo mucoadhesion time of all the formulations were evaluated after 1, 2, 3, 4, and 6 months intervals. The data collected as mean of three determinations. Stability of the selected buccal patches was determined in natural human saliva collected from healthy adult human volunteers aged between 35 to 40 years of either sex. Patches were placed in separate Petri dishes containing 5 mL of human saliva and kept in an incubator maintained at 37 ± 0.2 °C for 12 hours.
Fourier transform infrared (FTIR) spectra of selected CBZ buccal patch formulations (stored at 40 ± 2oC / 75% ± 5% RH for 2 months) were recorded by potassium bromide disc method. And the samples of same formulations were subjected to X-ray diffraction (XRD) studies. The Powder X-ray diffraction patterns were studied (Anton Paar, TTK 450 diffractometer, Austria) to know the physical form of drug and polymers used in the formulations. The X-ray generator was set at 40kV and 35mA and configured at 2θ geometry.
A total of 36 formulations of buccal patches of CBZ were prepared using a 32 full factorial design using mucoadhesive polymers CH/HPMC/PVA and CH/HPMC/CP. Propylene glycol or PEG 400 was used as plasticizer. The patches were characterized for their thickness, folding endurance, mass uniformity, drug content uniformity, surface pH, percentage swelling and mucoadhesive properties (Table 2).
The film thicknesses were observed in the range of 0.3 ± 0.10 and 0.5 ± 0.15 mm and mass of 3 cm diameter patches was ranged from 142.4 ± 0.22 and 160.2 ± 0.13 mg.
The results of drug content uniformity showed that the drug was uniformly dispersed. The patches showed sufficient drug loading which varied between 18.1±0.5 and 19.8±0.1 mg per 3 cm diameter patch.
The pH of the prepared patches was slightly acidic and ranged between 4.9±0.01 to 6.2±0.06 and this may be due to citric acid used in the formulation to dissolve chitosan. But this pH range is close to salivary pH and there was no mucosal irritation expected.
Out of 36 formulations, five formulations (FA42, FA46, FB38, FB49 and FB52) showed high folding endurance of ˃255. These patches were selected for further evaluation such as percentage of swelling, mucoadhesion and drug release studies.
The percentage swelling was increased in the following order, FA42 < FB38 < FA46 < FB52 < FB49. The difference in swelling of the hydrophilic polymers may be due to the difference in resistance of matrix network structure to the movement of water molecule.The swelling property of the polymer was reported to be fundamental for its mucoadhesive strength. The weak adhesion occurs immediately after the swelling was initiated but the strength of mucoadhesion increases with the degree of hydration to a certain point. But over hydration results a sudden drop in adhesive strength due to disentanglement at the polymer mucosa intersection. The rate and the extent of swelling of patches influences mucoadhesion and also the drug release rate from the patches. Fig 1 shows the percentage of swelling of patches in simulated saliva solution pH 6.2. Higher percentage of swelling was observed with formulations containing HPMC and CP in higher proportion (FB52, FB49, and FA46). The percentage of swelling was higher up to 53 ± 1.1 for FB49 after 160 min. The plasticizer PEG 400 containing patches showed increased water uptake and swelling compared to propylene glycol. The presence of CH and PVA in formulations decreases the rate and extent of swelling. The increased swelling may promote more drug release but due to increase in diffusion path the rate of release may be delayed. However the thick swollen layer formed around the patch matrix may prevent quick disintegration. Although the swelling was high, none of the patches illustrate any significant variation in their nature.
The mucoadhesion time and mucoadhesive strength of the selected formulations is shown in Fig 2. The ex vivo mucoadhesion time of selected patches was ranged between 112±3.9 to 167±2.3 min. None of the patches were detached throughout the study period from the mucosal membrane and this indicated the duration required to retain the patch on the mucosal membrane during drug release. The ex vivo mucoadhesive force of the selected formulations was recorded in the range of 0.285 to 0.512 Kg.m.s-2. The highest mucoadhesive force was observed with formulation FA42 which is containing chitosan in higher proportion. The presence of cationic polymer chitosan shows very high mucoadhesive strength on mucosal surface. Decreased mucoadhesive strength was observed with the patches composed of HPMC and CP due to hydrophilicity and swelling. This weakens the integrity of polymer matrix and lead to erosion of swollen layer. The increased porous surface allows diffusion of external fluid. High water uptake of PEG-400 used patches shows increased mucoadhesion due to increased interpenetration of polymer and mucin chain at the interface.
The In vitro releases of the selected CBZ buccal patches are illustrated in Fig 3. In vitro release revealed that formulation FB49 showed maximum release of 99.9±1.0 after 90 min and followed by FB52 (105 min), FA46 (135 min), FA42 (150 min) and FB38 (150 min). From the In vitro release studies it was concluded that the patches prepared with PEG-400 showed maximum release while compared with those patches prepared with PG as plasticizer. Presence of carbopol increased drug release rate than PVA from chitosan-HPMC matrix patches. The drug release rate may be correlate with increased swelling observed in CP patches that may weakens the integrity of polymer matrix and lead to erosion of swollen layer. Initially all the patches showed an erratic drug release and were not ideal for a controlled drug delivery system [11].
The mechanism of drug release indicated that the formulations FA42, FA46, FB38, FB49 and FB52 were best fitted to Korsmeyer-Peppas model. The Table 3 showed the R2, k and n values of the selected formulations. The release exponent value of ‘n’ characterizes the transport mechanism of drug as described in Table 3. All selected formulations the value of n was greater than 0.89 that indicates super case II drug transport that refers drug release by erosion of the polymeric chain.
The results of ex vivo permeation of CBZ from selected patches (Fig 4) indicated that the drug is released and permeated through the porcine buccal mucosa, thus would have permeated through human mucosal membrane also. There was a good correlation between In vitro drug release and ex vivo permeation studies. The R2 for the formulation FA42 was 0.9987, FA46 (0.999), FB38 (0.9991), FB49 (0.9977) and FB52 (0.9997 [12].
The FTIR spectra and XRD of the selected formulation (FB33) is shown in Fig 5. The spectra of formulation FB49 showed characteristic peaks of CBZ at 3602.53, 3013.34, 1678.87, 1599.72 and 1497.57 cm-1 were recorded due to N-H, C-H, C=O and C=C stretching respectively. The spectra obtained from the formulations showed all the principle peaks are at or around the requisite wave number of the pure drug. This confirmed the purity and integrity of the drug in the formulation.
The XRD pattern of formulation FB49 showed less intensity and reduced number of peaks than powder form of individual components. This change indicates a reduction in crystallinity and increase in amorphous nature. This may be due to increased solubility of components in the formulation matrix. The distinctive peaks of CBZ at 13.52º(2θ), 15.73º(2θ), 25.34º(2θ) and 27.77º(2θ) revealed that the drug is present in crystalline state in the formulation.
The accelerated stability study showed that the drug content of the patches were ranged between 19.5 ± 3.8 and 19.8 ± 1.2 mg. Mucoadhesion time of patches showed between 110 ± 2.6 to 167 ± 2.0 min (Table 4). During and at the end of the accelerated stability study tested patches showed similar drug content, mucoadhesion time and surface pH. Stability studies conducted in normal human saliva shows no abnormal color changes or changes in the texture.
Unidirectional mucoadhesive buccal patches of carbamazepine were developed to improve the bioavailability by avoiding the hepatic first pass metabolism and thereby reduce metabolite dependent adverse drug effect. The In vitro release profile reveled that maximum amount of carbamazepine is released from the prepared patches within specified mucoadhesion period and this indicated that the prepared novel unidirectional mucoadhesive buccal patches of carbamazepine would be a potential drug delivery system for systemic delivery of carbamazepine. It can be conclude that chitosan buccal adhesive patches can be successfully used as a carrier in buccal drug delivery systems for CBZ which undergo first-pass metabolism. But in future this has to be confirmed with In vivo studies.