ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Janani K., Ketzi M., Megavathi S.1, Dr.Vinothkumar D.2, Dr. Ramesh Babu N.G.3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Production of Ethanol fermented from renewable sources for fuel or fuel additives are known as bioethanol. Since the need of bioethanol has been increasing, the production of bioethanol must be increased using cheaper and eco friendly raw materials. On the basis of these characteristics fruit wastes can be considered as cheaper and eco friendly. In this study different fruit wastes were used as a raw material for the production of bioethanol by using Saccharomyces cerevisiae and the result were compared. The results of this work shows that the rate of ethanol production through fermentation of grape fruit waste by Saccharomyces cerevisiae (baker’s yeast) yields is very at pH 5.4, temperature 30°C, specific gravity 0.872,concentration of about 6.21% than other fruit wastes. The results of this study suggest that wastes from fruits that contain fermentable sugar should not be discarded into our environment, but should be converted to useful products like bioethanol that can serve as an alternative energy source
Keywords |
| Saccharomyces cerevisiae, fermentation, pH, temperature, concentration, specific gravity. |
I. INTRODUCTION |
| The largest single use of ethanol is as a motor fuel and fuel additive. It is also an important industrial ingredient and has widespread use as a base chemical for other organic compounds and used in medical wipes and most commonly as an antibacterial hand sanitizer gels and as an antiseptic. Additionally, ethanol from biomass-based waste materials is considered as bioethanol [1]. Alcohol is an organic compound that has one or more hydroxyl (OH) groups attached to a carbon atom. In dilute aqueous solution, it has a somewhat sweet flavor, but in more concentrated solutions it has a burning taste [2]. Ethanol fermented from renewable sources for fuel or fuel additives is known as bioethanol. Bioethanol, unlike petroleum, is a form of renewable energy source that can be produced from agricultural feedstocks [3]. The first generation of ethanol production used corn as a substrate, later corn was considered as a feedstock lead to the second generation of production of ethanol which used microorganisms and different wastes as substrates [4]. |
| The cheapest and easily available source for the production of bioethanol is fruit wastes. It is a potential energy source, from which ethanol can be obtained. Fruitwaste which is thrown away has very good antimicrobial and antioxidant potential. In this study, comparing of the ethanol efficiency produced by fermentation process from different fruit wastes such as papaya, grapes, apples and bananas. The efficiency of the ethanol can be calculated by determining the various parameters such as pH, temperature, specific gravity and concentration. Saccharomyces cerevisiae is used in the fermentation process since it converts sugar with oxygen to give carbon dioxide. |
| Carica papaya (pawpaw) is one of the common fruits available with the highest source of energy and invert sugar. So papaya fruit wastes can be used for the production of bioetahnol by fermentation process. Grapes are the fruit of a wine (Vitis vinifera). The whole fruit, skin, leaves and seed of the grape plant are used as medicine. Grape wastes can be used as a substrate for the production of ethanol. The yield of ethanol amounted to greater than 80% of the fermentable sugar consumed [5]. Although waste bananas (Musa paradisiaca) are commonly discarded as waste they represent a potential energy feedstock which may be especially suited for ethanol production. Waste bananas are low cost, concentrated biomass feedstock. Apple (Malus pumila) is the oldest fruit known to man and is grown extensively throughout the temperate zones of the world [6]. Apple is also one of the good sources for the production of ethanol.The main objective of this study is compare the ethanol efficiency obtained from different fruit waste |
II. MATERIALS AND METHODS |
Materials and Equipments |
| The chemicals are that used in this research are 5% potassium permanganate (KMnO4), 50g sucrose, 1g urea, Saccharomyces cerevisiae strains, different fruit wastes such as apple, banana, papaya and grapes. The equipments used are hot plates, incubators, distillation unit and hydrometer. |
Preparation of the substrate for the fermentation process |
| About 200g of Fruit wastes such as apple, banana, papaya and grapes (Fig 1) were weighed separately and were taken in different beakers, which was washed with 5% potassium permanganate solution and then rinsed with distilled water. Apple waste, banana waste and grape waste, papaya waste were crushed separately in a mixer and collected in beakers |
 |
| 1g of urea, 50g of sucrose and 10g of Saccharomyces cerevisiae was mixed well in warm water in a separate beaker. This mixture was taken as an inoculum for fermentation process for one fruit mash. The fruit mash and inoculum were transferred into 1.5 liter conical flask and made upto the final volume 1000ml with distilled water. The sample is subjected to the fermentation process. The same procedure was followed for other fruit mashes for the preparation of the sample. The initial specific gravity of the mixture was determined by using hydrometer. |
| These flasks containing different fruit waste samples were kept in an incubator at 36°C. During fermentation process, zymase an enzyme from yeast changes the simple sugars into ethanol and carbon dioxide. During incubation, specific gravity of the sample was noted frequently by using a hydrometer. When the specific gravity reaches a steady value, it indicates the end of the fermentation process. The incubation period varies for each fruit waste sample. |
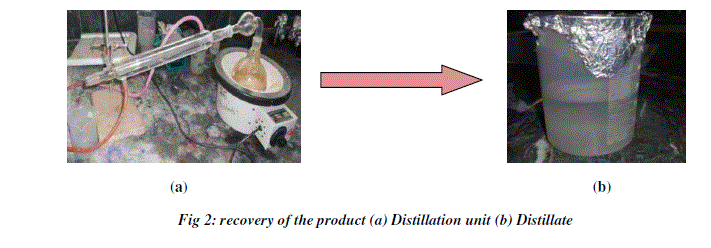 |
| After the fermentation process a small amount each sample was taken and centrifuged. The supernatant was collected and the volume of the alcohol was determined by the specific gravity method. Then the rest of the sample was distilled using simple distillation unit to collect the ethanol from different fruit wastes. For distillation, the batch distillation method was adopted (Fig 2). The distillation unit consisted of three components: a reboiler, condenser pipe and a distillate or receiving flask. The filtered sample was transferred into the reboiler and the sample was boiled. The vapors started to rise into the still head and passed through the condenser pipe. The continuous circulation of cold water around the condenser pipe assisted in cooling the alcohol rich vapors back to the liquid state. The condensed liquid enters the still receiver and is then collected in the distillate. The final tests such as pH, temperature, specific gravity and concentration of bioethanol were determined. The amount of ethanol obtained from different fruit wastes was recorded and the solution was subjected to iodine test to confirm the presence of ethanol. |
III. RESULTS AND DISCUSION |
| In this study, it has been shown that ethanol was produced from different fruit wastes. Thus the comparative study has been carried out to check the efficiency of ethanol produced from these fruit wastes by determining various parameters. The influence of various parameters on the production of ethanol is presented as follows |
Effect of temperature |
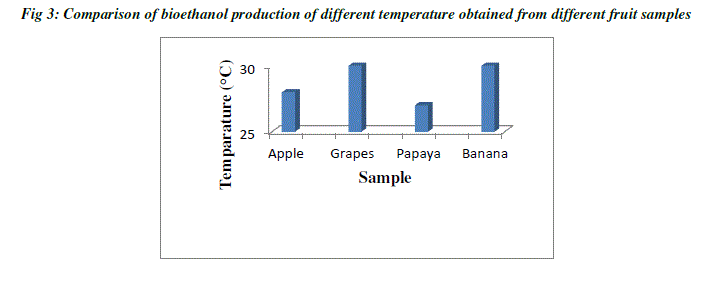
| Temperature plays a major role in the production of ethanol, since the rate of alcoholic fermentation increases with the increase in temperature. The optimum temperature of ethanol ranges between 25°C to 40°C which depends on room temperature. Bioethanol produced from both grapes and banana have same temperature of about 30°C, and ethanol from apple has 28°C temperature, whereas ethanol from papaya has 27°C. When temperature goes below the optimal range, their ability to catalyze the intended reaction slows down (Table 1; Fig3). On the other hand, when the temperature increases, enzymes begin to denature or unfold and thus become inactive. Each enzyme will have a different temperature range where it becomes inactive. Even if one essential enzyme stops working, the organism fails to grow. Hence, the first essential enzyme that gets deactivated defines the maximal temperature at which that organism can grow. At the lower end, it gets more complicated. Usually, the enzymes are not inactivated but rather just slow down [7] |
 |
 |
Effect of pH |
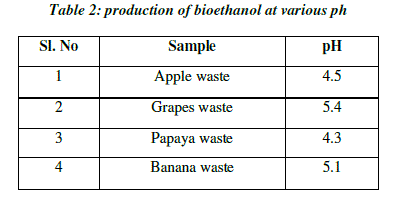
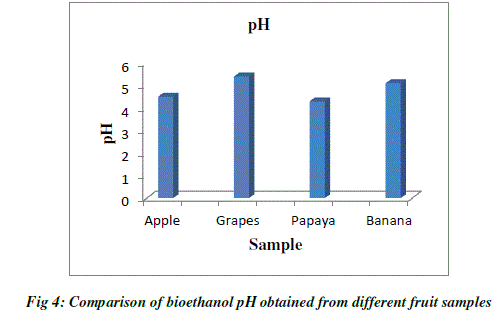
| pH value has significant influence on alcoholic fermentation. pH of bioethanol produced from different fruit wastes were determined. The pH value of ethanol produced by the process of fermentation ranges from 4 to 6. Yeast survives in a slightly acidic environment that is with pH between 4 to 6 .The pH value of ethanol obtained from fruit wastes was determined as, grape at (pH=5.4), apple (pH=4.5), papaya (pH=4.3) and banana (pH=5.1). Among these ethanol produced from grape wastes had on higher alcoholic content (Table 2; Fig.4). |
 |
 |
 |
Effect of specific gravity |
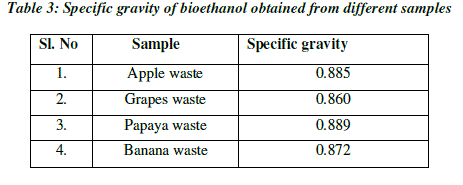
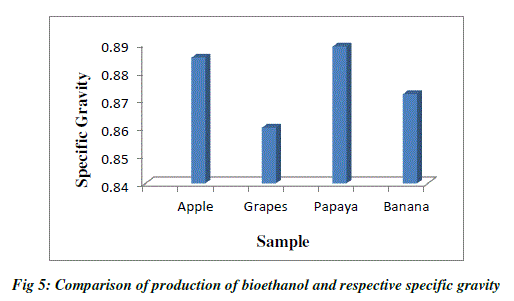
| Specific gravity is used to measure the sugar content. As the fermentation progressed, the specific gravity considerably decreased and reached a value of 0.860 at 48hrs and remained constant. The specific gravity of apple after fermentation was reduced to 0.885 at 36hrs and remained constant. The specific gravity of papaya after fermentation was reduced to 0.872 at 72hrs, whereas the specific gravity of banana was reduced to 0.834 at 72hrs. The decrease in specific gravity is clear indication of yeast fermenting the sugar resulting in ethanol production. The specific gravity reaching a constant value after incubation period is the indication of end of fermentation (Table 3; Fig.5). |
 |
 |
Effect of concentration |
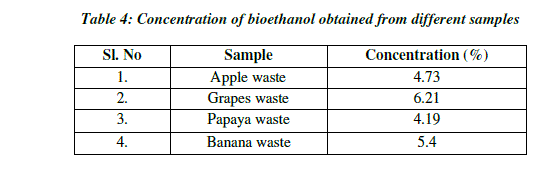
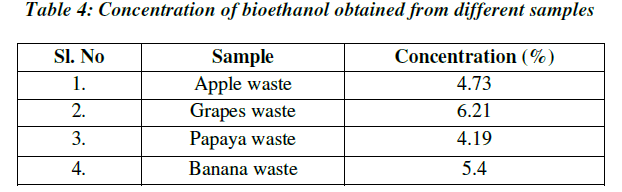
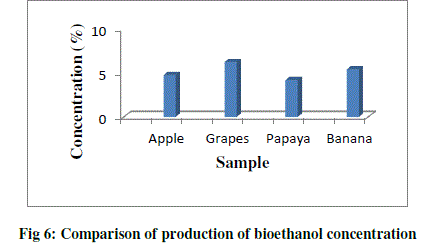
| Concentration is the measure of ethanol content present in the distillate. Concentration of ethanol was expressed in terms of percentage (%). The concentration of ethanol present in the grape sample was 6.21% and the ethanol in the papaya sample was 4.19%, whereas the concentration of ethanol present in the apple sample was 4.72% and ethanol present in banana was 5.40%. With the increase in sugar concentration, the ethanol production increased significantly (Table 4; Fig.6). |
 |
 |
| The result of this work has shown that different fruit wastes can serve as raw material for the production of bioethanol. From this comparative study, it is clear that the maximum yield of ethanol was obtained from grape wastes at pH 5.4, temperature 30oC, specific gravity 0.860, and concentration of 6.21% which is appropriately close the constant value of ethanol. Finally, comparative studies of bioethanol production from different fruit wastes shows that grape waste has higher efficiency than other fruit wastes such as apple and banana, papaya or mixed fruit wastes. This process is cost effective and does not yield any toxic residues. Hence, it can be run as a small scale industry. |
ACKNOWLEDGEMENT |
| The authors thank to the Chairman and the Principal of Adhiyamaan College of Engineering, Hosur for providing laboratory facilities to carry out this investigation. |
References |
|