ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
K. Kishor1 M.V.S. Murali Krishna2 and P.V.K. Murthy3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Experiments were conducted to evaluate the performance and control the exhaust emissions from four stroke, variable speed, variable compression ratio, single cylinder, spark ignition (SI) engine, with alcohol blended gasoline (80% gasoline, 10% methanol, 10% ethanol by volume) having copper coated combustion chamber [CCCC, copper-(thickness, 300 μ) coated on piston crown, inner side of cylinder head] provided with catalytic converter with sponge iron as catalyst and compared with conventional SI engine (CE) with pure gasoline operation. Performance parameters brake thermal efficiency, exhaust gas temperature and volumetric efficiency) and exhaust emissions (carbon monoxide (CO) and un-burnt hydro carbons (UBHC)) were determined with different values of brake mean effective pressure of the engine. A microprocessor-based analyzer was used for the measurement of CO/UBHC in the exhaust of the engine. Copper coated combustion chamber with alcohol blended gasoline considerably improved the performance and reduced pollutants in comparison with CE with pure gasoline operation. Catalytic converter with air injection significantly reduced pollutants with test fuels on both configurations of the combustion chamber. The catalyst, sponge reduced the pollutants effectively with both test fuels in both versions of the combustion chamber.
Keywords |
| S.I. Engine, CE, copper coated combustion chamber, Performance, Exhaust Emissions, CO, UBHC, Catalytic converter, Sponge iron, Air injection |
I. INTRODUCTION |
| The paper is divided into i) Introduction, ii) Materials and Methods, iii)Results and Discussions, iv) Conclusions, Research Findings, Future scope of work followed by References. |
| This section deals with need for alternate fuels, important substitutes for gasoline, emissions from SI engine, their formation, effect of pollutants on human health, their impact on environment, change of fuel composition and engine modification to reduce pollutants and improve the performance, methods of reducing pollutants, catalytic converter, research gaps, objective of the experimentation. |
| The civilization of a particular country has come to be measured on the basis of the number of automotive vehicles being used by the public of the country. The tremendous rate at which population explosion is taking place imposes expansion of the cities to larger areas and common man is forced, these days to travel long distances even for their routine works. This in turn is causing an increase in vehicle population at an alarm rate thus bringing in pressure in Government to spend huge foreign currency for importing crude petroleum to meet the fuel needs of the automotive vehicles. The large amount of pollutants emitting out from the exhaust of the automotive vehicles run on fossil fuels is also increasing as this is proportional to number of vehicles. In view of heavy consumption of petrol due to individual transport, and fast depletion of fossil fuels, the search for alternate fuels has become pertinent apart from effective fuel utilization which has been the concern of the engine manufacturers, users and researchers involved in combustion & alternate fuel research. |
| In the context of fast depletion of fossil fuels, the search for alternate fuels has become pertinent. Alcohols are probable candidates as alternate fuels for SI engines, as their properties are compatible close to gasoline fuels. If alcohols are blended in small quantities with gasoline fuels, no engine modification is necessary. |
| Carbon monoxide (CO) and un-burnt hydrocarbons (UBHC), major exhaust pollutants formed due to incomplete combustion of fuel, cause many human health disorders [1-2]. These pollutants cause asthma, bronchitis, emphysema, slowing down of reflexes, vomiting sensation, dizziness, drowsiness, etc. Such pollutants also cause detrimental effects [3] on animal and plant life, besides environmental disorders. Age and maintenance of the vehicle are some of the reasons [4-6] for the formation of pollutants. |
| Engine modification [7-9] with copper coating on piston crown and inner side of cylinder head improves engine performance as copper is a good conductor of heat and combustion is improved with copper coating. The use of catalysts to promote combustion is an old concept. More recently copper is coated over piston crown and inside of cylinder head wall and it is reported that the catalyst improved the fuel economy and increased combustion stabilization. |
| Catalytic converter is one of the effective [10-14] methods to reduce pollutants in SI engine. Reduction of pollutants depended on mass of the catalyst, void ratio, temperature of the catalyst, amount of air injected in the catalytic chamber. A reduction of 40% was reported with use of sponge iron catalyst while with air injection in the catalytic chamber reduced pollutants by 60%. |
| Alcohol was blended [15-17] with gasoline to reduce pollutants and improve the performance. CO and UBHC emissions reduced with blendes of alcohol with gasoline. |
| The present paper reported the performance evaluation of copper coated combustion chamber with alcohol blended gasoline (gasoline-80%, methanol-10% and ethanol-10% by volume) and compared with pure gasoline operation on CE. Exhaust emissions of CO and UBHC were controlled by catalytic converter with sponge iron as catalyst. |
II. MATERIALS AND METHODS |
| This section deals with fabrication of copper coated combustion chamber, description of experimental set up, operating conditions of catalytic converter and definition of used values |
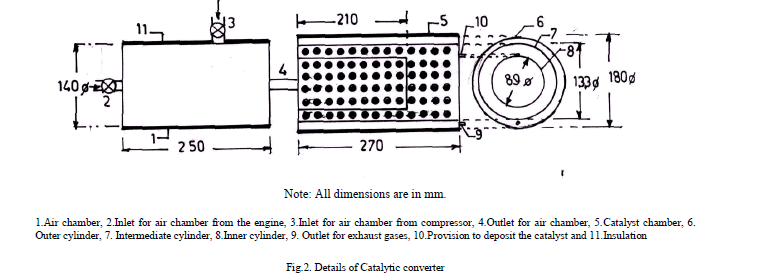
| In catalytic coated combustion chamber, crown of the piston and inner surface of cylinder head are coated with copper by flame spray gun. The surface of the components to be coated are cleaned and subjected to sand blasting. A bond coating of nickel- cobalt- chromium of thickness 100 microns is sprayed over which copper (89.5%), aluminium (9.5%) and iron (1%) alloy of thickness 300 microns is coated with METCO flame spray gun. The coating has very high bond strength and does not wear off even after 50 h of operation [7]. |
| Figure.1. shows schematic diagram for experimental set-up used for investigations. A four- stroke, single-cylinder, water-cooled, SI engine (brake power 2.2 kW, rated speed 3000 A rpm) was coupled to an eddy current dynamometer for measuring brake power. Compression ratio of engine was varied (3 -9) with change of clearance volume by adjustment of cylinder head, threaded to cylinder of the engine. Engine speeds are varied from 2400 to 3000 rpm. Exhaust gas temperature is measured with iron- constantan thermocouples. Fuel consumption of engine was measured with burette method, while air consumption was measured with air-box method. The bore of the cylinder was 70 mm while stroke of the piston was 66 mm. The engine oil was provided with a pressure feed system. No temperature control was incorporated, for measuring the lube oil temperature. Recommended spark ignition timing was 25oaTDC. CO and UBHC emissions in engine exhaust were measured with Netel Chromatograph analyzer. |
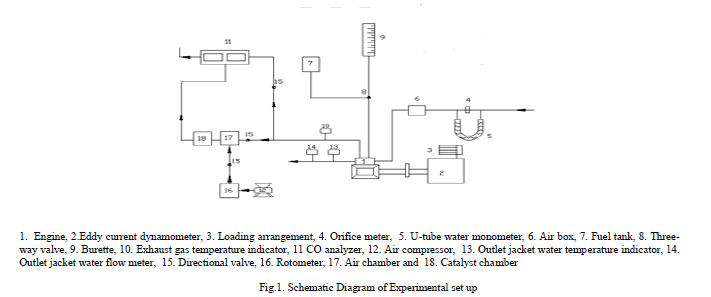 |
| A catalytic converter [11] (Figure.2) was fitted to exhaust pipe of engine. Provision was also made to inject a definite quantity of air into catalytic converter. Air quantity drawn from compressor and injected into converter was kept constant so that backpressure does not increase. Experiments were carried out on CE and copper coated combustion chamber with different test fuels [pure gasoline and alcohol blended gasoline (20% by vol)] under different operating conditions of catalytic converter like set-A, without catalytic converter and without air injection; set-B, with catalytic converter and without air injection; and set-C, with catalytic converter and with air injection. |
 |
| Definitions of used values: |
| Brake mean effective pressure: It is defined as specific torque of the engine. Its unit is bar. |
 |
III. RESULTS AND DISCUSSION |
| This section deals with determination of performance parameters and exhaust emissions, |
A. Performance Parameters |
| This section deals with i) variation of brake thermal efficiency, exhaust gas temperature, volumetric efficiency with brake mean effective pressure with test fuels with different versions of the combustion chamber, and ii) variation of brake specific energy consumption at full load operation with test fuels with various combustion chambers. |
| Figure.3 shows the variation of brake thermal efficiency (BTE) with brake mean effective pressure (BMEP) in different versions of the combustion chamber with test fuels of pure gasoline and alcohol blended gasoline at a compression ratio of 9:1 and speed of 3000 rpm. BTE increased up to 80% of the full load operation with an increase of BMEP in different versions of the combustion chamber, with different test fuels. Beyond 80% of the full load operation, BTE decreased with test fuels due to reduction of volumetric efficiency and air fuel ratio. Similar trends were observed by Murali Krishna [10]. Higher value of BTE was observed with alcohol blended gasoline over pure gasoline at all loads due to lower Stochiometric air requirement of alcohol blended gasoline over pure gasoline operation. |
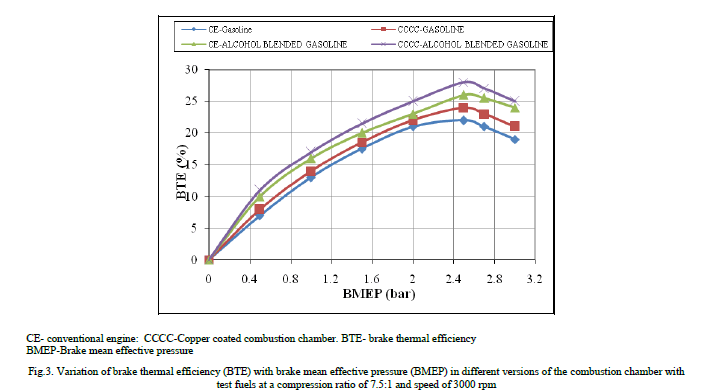 |
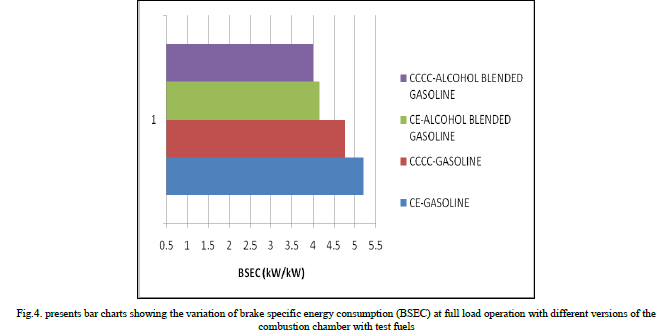
| Copper coated combustion chamber showed higher thermal efficiency when compared to CE with both test fuels at all loads, particularly at near full load operation, due to efficient combustion with catalytic activity, which was more pronounced at peak load, as catalytic activity increases with prevailing high temperatures at full load. Figure.4 presents bar charts showing the variation of brake specific energy consumption (BSEC) at full load operation with different versions of the combustion chamber with test fuels. Copper coated combustion chamber showed lower BSEC in comparison with CE with test fuels. This was due to improved combustion with increased catalytic activity with test fuels. Alcohol blended gasoline showed lower value of BSEC in comparison with pure gasoline operation on both versions of the combustion chamber. This was due to lower theoretical air fuel ratio requirement of alcohol blended gasoline so as to improve combustion with improved air fuel ratios. |
 |
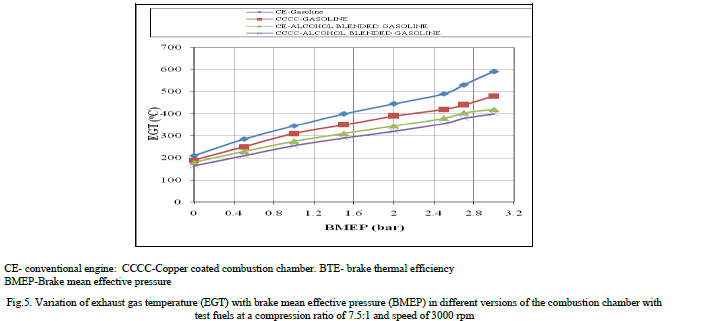
| Figure.5 shows the variation of exhaust gas temperature (EGT) with BMEP in different versions of the combustion chamber with test fuels at a speed of 3000 rpm and compression ratio of 9:1, which indicated that EGT increased with an increase of BMEP. This was due to increase of fuel consumption with load. The value of EGT was lower with alcohol blended gasoline when compared to pure gasoline at all loads in CE and copper coated combustion chamber, because, with alcohol blended gasoline, work transfer from piston to gases in cylinder at the end of compression stroke was too large, leading to reduction in the value of EGT. This was also due to high latent heat of evaporation of alcohol blended gasoline. Copper coated combustion chamber registered lower value of EGT when compared to CE for both test fuels, which confirmed that efficient combustion with the copper coated combustion chamber in comparison with CE. |
 |
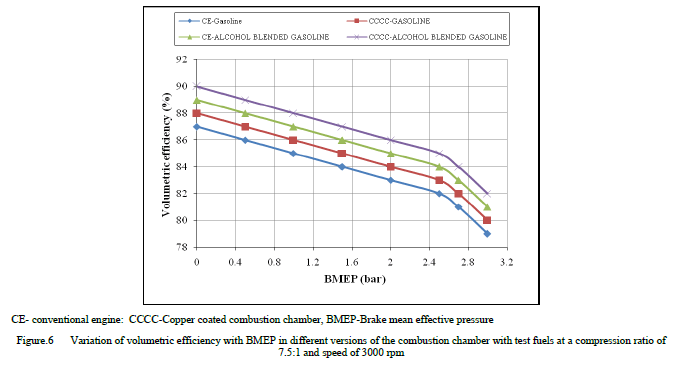
| Figure 6 shows the variation of volumetric efficiency (VE) with BMEP with test fuels at a speed of 3000 rpm and a compression ratio of 9:1, which indicated that volumetric efficiency decreased with increase of BMEP due to increase of gas temperature [10]. Copper coated combustion chamber showed marginally higher volumetric efficiency at all loads in comparison with CE with different test fuels, due to reduction of residual charge and deposits in the combustion chamber of CCE when compared to CE, which shows the same trend as reported earlier[14]. This was also because of lower value of exhaust gas temperatures as volumetric efficiency depends of temperature of combustion chamber walls. Volumetric efficiency increased marginally with alcohol blended gasoline when compared with pure gasoline operation with both versions of the combustion chamber at all loads, due reduction of temperature of air because of high latent heat of evaporation of alcohol blended gasoline. |
 |
B. Exhaust Emissions |
| This section deals with variation of CO emissions and UBH emissions with BMEP. This also contains data of CO and UBHC emissions at different operating conditions of the catalytic converter. |
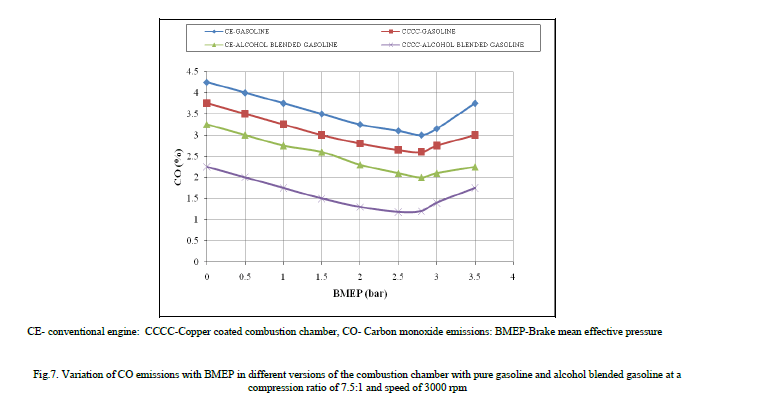
| Figure 7 shows the variation of CO emissions with BMEP in different versions of the engine with both pure gasoline and alcohol blended gasoline. CO emissions decreased with alcohol blended gasoline at all loads when compared to pure gasoline operation on copper coated combustion chamber and CE, as fuel-cracking reactions [13] were eliminated with alcohol.. The combustion of methanol or ethanol produces more water vapor than free carbon atoms as methanol has lower C/H ratio of 0.25, while with ethanol 0.33, against 0.50 of gasoline. Methanol or ethanol has oxygen in its structure and hence its blends have lower stochiometric air requirements compared to gasoline. Therefore more oxygen that is available for combustion with the blends of methanol and gasoline, leads to reduction of CO emissions. Methanol or ethanol dissociates in the combustion chamber of the engine forming hydrogen, which helps the fuel-air mixture to burn quickly and thus increases combustion velocity, which brings about complete combustion of carbon present in the fuel to CO2 and also CO to CO2 thus makes leaner mixture more combustible, causing reduction of CO emissions. |
| Copper coated combustion chamber reduced CO emissions in comparison with CE. Copper or its alloys acts as catalyst in combustion chamber, whereby facilitates effective combustion of fuel leading to formation of CO2 instead of CO. Similar trends were observed with Reference [7] with pure gasoline operation on copper coated combustion chamber. |
 |
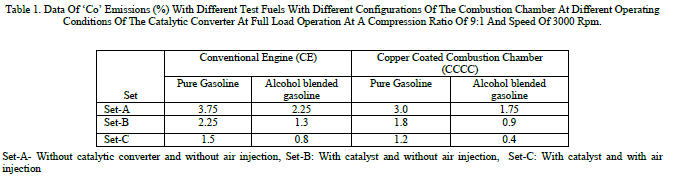
| Table 1 shows the data of CO emissions with different test fuels with different configurations of the combustion chamber at different operating conditions of the catalytic converter with different catalysts. From the table, it can be observed that CO emissions deceased considerably with catalytic operation in set-B with alcohol blended gasoline and further decrease in CO is pronounced with air injection with the same fuel. The effective combustion of the alcohol blended gasoline itself decreased CO emissions in both configurations of the combustion chamber. CO emissions were observed to be higher with alcohol blended gasoline operation in comparison with pure gasoline operation in both versions of the combustion chamber at different operating conditions of the catalytic converter. This is due to the reason that C/H ratio of alcohol blended gasoline is lower in comparison with that of pure gasoline operation. |
 |
| Figure.8 shows the variation of un-burnt hydro carbon emissions (UBHC) with BMEP in different versions of the combustion chamber with both test fuels. UBHC emissions followed the same trend as CO emissions in copper coated combustion chamber and CE with both test fuels, due to increase of flame speed with catalytic activity and reduction of quenching effect with copper coated combustion chamber. |
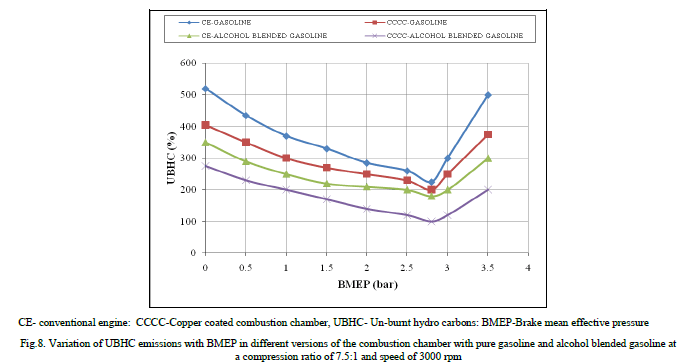 |
| Table 2 shows the data of UBHC emissions with different test fuels with different configurations of the combustion chamber at different operating conditions of the catalytic converter with sponge iron. The trends observed with UBHC emissions are similar to those of CO emissions in both versions of the engine with both test fuels. From Table, it is observed that catalytic converter reduced UBHC emissions considerably with both versions of the combustion chamber and air injection into catalytic converter further reduced pollutants. In presence of catalyst, pollutants further oxidised to give less harmful emissions like CO2. Similar trends are observed with Reference [7] with pure gasoline operation on CCE. |
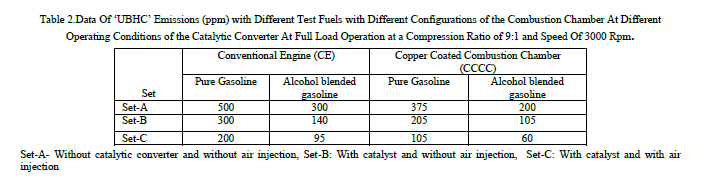 |
IV. CONCLUSIONS |
| 1. Thermal efficiency increased by 9% with gasoline operation, while with alcohol blended gasoline operation it increased by 8%. |
| 2. Exhaust gas temperature decreased by 19%, with gasoline operation, while with alcohol blended gasoline operation it decreased by 5%. |
| 3. Volumetric efficiencies were compatible with gasoline operation as well as alcohol blended gasoline operation. |
| 4. CO and UBHC emissions at full load operation decreased by 20% with CCE when compared with CE with both test fuels. |
| 5. Set-B operation decreased CO and UBHC emissions by 40%, while Set-C operation decreased these emissions by 60% with test fuels when compared to Set-A operation. |
| 6. Sponge iron is proved to be more effective in reducing the pollutants. |
A. Research Findings and Future Scope of Work |
| Investigations on control of exhaust emissions in two-stroke SI engine were systematically carried out. Spark plug timing can be varied to improve the performance further and reduce pollutants more effectively. |
ACKNOWLEDGEMENTS |
| Authors thank authorities of Chaitanya Bharathi Institute of Technology, Hyderabad for facilities provided. The financial assistance from Andhra Pradesh Council of Science and Technology (APCOST), Hyderabad, is greatly acknowledged. |
References |
|