1Centre for Health Economics Research (COHERE), Department of Public Health, University of Southern Denmark, J.B. Winsløwsvej 9B, 5000 Odense C, Denmark
2Hospital Pharmacy, Central Denmark Region, Nørrebrogade 44, 8000 Aarhus C, Denmark
3Research Centre for Emergency Medicine, Aarhus University Hospital, Building 30, Noerrebrogade 44, 8000 Aarhus C, Denmark
4Life Sciences Group, IP & Technology, Bech-Bruun law, Langelinie Alle 35, DK- 2100 Copenhagen, Denmark
5Health Outcome Research Centre, Royal College of Surgeons in Ireland, Beaux Lane House, Dublin 2, Ireland
Received date: 14/01/2017; Accepted date: 27/01/2017; Published date: 03/02/2017
Visit for more related articles at Research & Reviews: Journal of Hospital and Clinical Pharmacy
Objective: The risk of errors in the medication administration process is high. Applications of prefilled syringes may improve patient safety but could be more costly. The objective of this study was to assess the additional costs of a ready-to-use syringe delivery programme in comparison with a conventional delivery programme at day surgery and endoscopy departments at a large university hospital. Methods: The cost analysis used the hospital perspective and developed an “activity-based costing” model to assess the costs of medicinehandling activities. The model was calibrated with six-month data from a ready-to-use syringe delivery programme. Detailed measures of time and resource use related to the preparation process were obtained by direct observations. Registry-based data on activity, consumption and discards were obtained before and after the implementation to supplement the observed data. Local unit costs were converted to 2013-€ to estimate the incremental costs. Results: The analysis showed that the ready-to-use programme was more costly than the conventional delivery programme. The annual incremental cost for the day surgery department was estimated at €70,469 (an increase of 105%) and at €20,905 (an increase of 228%) for the endoscopy department. The ready-to-use delivery program imposed an additional cost of €11.32 per day surgery operation and €2.41 per endoscopy procedure. Conclusion: This ready-to-use programme increased the cost of the medical handling process. This incremental cost is likely to provide improvements in the quality of the administrative process, patient safety and staff satisfaction.
Cost analysis, Day surgery, Medication handling, Medication safety, Prefilled syringes, Ready-to-use
Effective and safe handling of medicine is of on-going concerns for many clinical and pharmaceutical managers. In their practice they seek to achieve high quality and patient safety at a reasonable cost. However, both the demand and cost of health care services are rising and there is more attention to ensure that services provided are cost-effective. When new means of improving quality are considered, it becomes important for managers to ensure that there is a reasonable balance between cost and quality improvement [1,2].
Previous studies of the handling and administration of injection medicines during surgical anaesthesia have indicated a need for further analysis of the cost and effectiveness. In 2010, Garguilo et al. highlighted the need to improve aseptic techniques during anaesthesia as a means to reduce the risk of medicine contamination and infections [3]. The frequency of errors in medication processes may be high during surgical anaesthetics. A study from 2001 found 1 error per 133 opportunities for errors during anaesthetics processes [4]. A 2013-review combined data on medication errors from three prospective studies and estimated a medication error rate of 1 error per 211 opportunities for errors [5]. They concluded that the error rate had not changed over recent years despite the fact that the cost consequences of medication errors may be substantial. They further recommended that more emphasis should be made to improve the quality and patient safety related to the anaesthetic processes [5].
Application of aseptically prepared medicine in prefilled syringes with clear and standardized colour-coded labels may be an important method to improve quality and patient safety during anaesthesia [6,7]. This is supported by the fact that easily recognizable syringes and visual identification reduce medication errors [7-10].
Cost impact analyses of applications of prefilled syringes in comparison with traditional procedures have shown different results. In 2000, Scheifele et al. found that prefilled syringes were associated with additional costs [11]. However, other studies have reported potential cost savings [12-15]. Two French studies evaluated the cost of administration of ephedrine by comparing ampoules/vials applications with prefilled syringes. Both studies found that prefilled syringes reduced the overall costs because they had longer durability and the need to discard unused medicine was reduced [16,17]. Most of the existing cost analyses have analysed handling costs for a single type of medicine. Only Webster et al. analysed the cost of a whole system of anaesthetic medicines that combined prefilled syringes, clear labelling, and bar-code scanning as part of a full, ready-to-use (RTU) syringe delivery programme [18]. Their analysis indicated that such a system was associated with a cost increase of €23 per anaesthetic procedure [18]. These examples show that different principles have been applied to analyse the cost of medicine delivery systems and that no robust method has yet been developed to enable more general cost assessments. As systematic cost analyses provide an important prerequisite for decisions about implementations of quality improvements in the medicine handling and administrative processes there is a need for a reliable and transparent framework for conducting cost analyses and actual applications that may provide reliable information to support such decisions.
The objective of this study was to develop a general framework for the cost analysis of the medicine handling and administrative processes, and apply this framework to conduct a cost analysis of a fully implemented, real-life, ready-to-use syringe-delivery programme at three different Danish public hospitals.
Cost Analysis – An Activity-Based Costing Model
Any cost analysis should be defined by the analytical perspective and the time horizon. In case of the cost of medicine handling and administrative processes it is reasonable to assume a hospital perspective and to focus on the resource use related to such processes. Cost analyses are often designed as to compare the difference in costs between two or more alternatives, and the result is presented as the incremental cost of the new alternatives in comparison with an alternative that is denoted as the comparative or baseline situation [1]. The principles of “activity-based costing” entail an identification of relevant activities that are clearly defined, reasonable standardised (resource homogenous) and have substantial influence on the overall cost in the considered situations. The cost of each of these activities should be based on the necessary resource use including staff time, consumables, physical space and equipment. To estimate the total cost each alternative must be defined in terms of the frequency that each of the specified activities occur. By aggregating the frequency and cost of each activity the total cost is calculated, and the incremental cost is derived as the difference in total cost of different alternatives.
The total cost of the medicine administration process in a time period is thus estimated as the product of the number of processes in the time period multiplied by the average cost of the process defined as the unit cost. Comparing the cost of different medicine administration strategies may therefore apply different unit cost to the same number of administration processes in order to provide total cost calculations that can be compared across alternatives. The unit costs of the administration processes thus “drives” the cost difference and is crucial to be specifed in a realistic and transparent way.
Relevant activities in relation to the medication preparation and administration process include the staff time for preparing medication, the compound, syringe and other consumables, necessary equipment for the preparation, as well as staff time and other costs for handling, transport and storage of the medication. A further relevant aspect is the proportion of prepared medication that has to be discarded due to expiry.
Study Design
This costing study used the described framework to compare the cost of two alternative real-life settings for the medicine handling and administrative processes – the implementation of the RTU syringe programme compared against the situation prior to the program implementation. The study included observation and analysis of staff time to prepare medicine and assessment of other relevant unit cost based on information available from the participating departments.
Common costs for both alternatives, e.g., costs of medicines outside the specified assortment and cost beyond the medication process were disregarded as they were assumed to be similar and therefore have no impact on the incremental cost of the implemented syringe programme. Costs relating to management and administration of the departments, cleaning, and rent of the physical space were also disregarded, as were costs of potential adverse events and consequences of medication errors.
Setting and Participants
The cost analysis included four day surgery and two endoscopy departments from three different Danish public hospitals. These departments perform uncomplicated planned surgery for patients in generally good health. All departments were opened during the daytime, Monday to Friday. The departments were organized with a management and anaesthetic staff that served different medical specialties of the hospital. Each department had a preoperative assessment area, operation rooms with surgical equipment, and a patient recovery area. The day surgery departments used a medicine trolley containing relevant anaesthetic equipment, medicines, labels, and a small table that functioned as the anaesthetist’s workplace. The majority of patients were discharged a few hours after surgery.
The departments were characterised by a high number of programmed one-day procedures and a high daily use of injective medicine. The day surgery departments apply many different injectable medicines, while the endoscopy departments only used two different medicines in high quantities. These circumstances made it possible to assess the incremental cost of the RTU program with both a wide and narrow assortments.
Among the participating departments were one day surgery department and one endoscopy department selected as test sites for implementation of the RTU programme. The other four departments participated as controls and ensured that the data collection was conducted in different department and observations made in different settings. Prior to the data collection it was ensured by interviews and observational visits that all participating departments followed the regional standard procedures for handling and preparing syringes for surgery and anaesthesia.
At all departments’ nurses prepared “trollies” with syringes for each operating room. All syringes were prepared according to regional guidelines and should be clearly labelled with the name of the medicine, date and time of preparation, and initials of the nurse. Many of the syringes needed during the day were prepared in the morning and were stored in the trolley. Additional syringes were prepared during the day. With the exception of remifentanil, all unused, prepared syringes had to be discarded at the end of the day, due to their limited effectiveness and stability.
The RTU Syringe Delivery Programme
The RTU syringe programme was implemented in May 2013 for a period of six months. The basic features of the programme included:
• The hospital pharmacy prepared daily for each operating room a customized medicine box containing the expected use of prefilled syringes with: Propofol, remifentanil, ephedrine, fentanyl, atropine, suxamethonium, dexamethasone, alfentanil, ketorolac, ondansetron, lidocaine-epinephrine, bupivacaine and bupivacaine-epinephrine.
• Prefilled syringes with alfentanil and midazolam were delivered to the endoscopy department.
• The hospital pharmacy production unit prepared all syringes using semi-automatical equipment under aseptic conditions.
• All prefilled syringes had colour-coded labels and clear letters that complied with the standards adopted in several countries and recommended by the European Board of Anaesthesiology in 2008 and by the Helsinki Declaration for Patient Safety in Anaesthesiology in 2010 [9].
• The RTU medicine boxes were re-packed daily at the department by a clinical pharmacist. This allowed changes in the content of the boxes during the intervention period as well as for reporting the discards into the pharmacy register.
The shelf life of the medication in the RTU-program was tested by the Quality Department in the hospital pharmacy in collaboration with an external company. The specific medication, syringe size and shelf life are further described in Supplementary File 1.
Ethics
This study was registered and approved by the Danish Data Protection Agency (Journal no. 1-16-02-163-12). According to Danish law approval from the National Committee on Health Research Ethics was not required.
The Costing Model
All identified cost items were included in the following general costing framework for analysing the cost of each alternative.
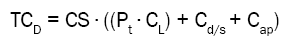
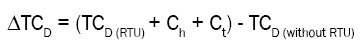
Total cost per medicine = Consumption ∙ ((Preparation time ∙ Labour costs) + Medicine/Syringe costs + Additional Production costs)
Total cost difference = (Total costs of all medicines (RTU) + Handling costs + Transportation costs) - Total costs of all medicines (without RTU)
Total costs were calculated for each different medicine and syringe size in the RTU assortment, with or without the RTU programme. The cost difference between the two alternatives was calculated (with and without RTU), summarized for all medicines included in the RTU assortment (TCD), with the addition of costs related to handling and transportation (Ch and Ct). Total costs and cost differences were calculated for a three-month period (13 weeks) and extrapolated to a year with 46 working weeks (the annual weeks with full time activity).
Measurement of Costs
Data on the consumption (CS) of medicine were obtained from the administrative pharmacy system as exact numbers of delivered medicines/prefilled syringes to each department. Data were obtained for a 3 month period during August 2013 and November 2013. Application of registry-based data ensured that similar data were available for the before and after comparison of costs.
Data on discarded medicine were obtained from the pharmacy register as numbers of discarded medicines reported for each department by the clinical pharmacist. The number of used syringes was calculated as the number of delivered medicine with subtraction of the reported number of discards. The registration of discarded medicine included only “full” syringes. Waste of residual medicines was expected to be similar with and without RTU and not considered in the analysis. The discard rate was calculated as the ratio between the discarded and used amount of medicine and is presented in Supplementary File 2. At the departments without RTU the discarded medicines included emergency medicines only, as these medicines were prepared in the operating room “just in case”. Other types of medicines were not prepared unless they were needed during the surgery.
The time nurses spent on syringe preparation (preparation time Pt) was obtained by direct observation by the same observer (BWR). Observations were made at all participating day surgery and endoscopy departments independently of whether they should implement the RTU program or not. A formal protocol for the observations was devised to ensure that the data collection followed a standardised procedure and to reduce variations due to observational differences. The observation time was defined to exclusively include time of preparing the parenteral medicines/syringes. The protocol included factors that were expected to have impact on preparation time including the gender, age, and years of working experience of the nurse/pharmacist. A wide range of variation in these factors was considered important for valid estimates of mean preparation time. All observed preparation times from the different departments were pooled and estimates of mean preparation time were calculated. Injectable medicines were delivered in different forms (ampoules, vials, or solids) and the volume of syringes prepared at the departments ranged from 1 ml to 50 ml. We calculated mean preparation time for four injection groups in the day surgery department and two groups in the endoscopy department based on medicines that were shown to require similar preparation time. The mean preparation time per group is presented in Table 1.
| Injection group | Group content | N | Mean time (s) | SD | Min | Max | Median |
|---|---|---|---|---|---|---|---|
| Day surgery department | |||||||
| Group 1 | Propofol, volume 20 & 50 ml | 52 | 67 | 27.12 | 27 | 184 | 61 |
| Group 2 | Remifentanil, volume 20 & 50 ml | 40 | 111 | 39.85 | 59 | 246 | 105.5 |
| Group 3 | Delivery form: ampoule, volume 1 & 2 ml | 159 | 54 | 20.08 | 19 | 112 | 51 |
| Group 4 | Local anaesthetics (vials), volume 10 & 20 ml | 28 | 43 | 18.94 | 22 | 104 | 37 |
| Endoscopy department | |||||||
| Group 1 | Alfentanil, volume 2 ml | 41 | 58 | 18.40 | 37 | 102 | 53 |
| Group 2 | Midazolam, volume 5 ml | 38 | 66 | 15.03 | 49 | 119 | 67 |
Table 1: Summary statistics of the preparation time in the clinical department (in seconds) per syringe by medicine group and content.
Also the time that staff from the hospital pharmacy used to prepare prefilled syringes were obtained by observation (preparation time). Prefilled syringes were prepared in batches at special production facilities at the hospital pharmacy and the staff time used relate both to the actual production and to quality control and assurance. A similar observational protocol was use to observe the duration from the time where the pharmaceutical staff entered the sterile production area until they had completed a full bath of medications. The average syringe preparation time was calculated as the batch production time divided by the batch size. The mean preparation times for the different medicines are presented in Table 2.
| Batch size | Preparation time per batch (hours, h) | Staff costs of preparation time per batch (2013-€) |
||||
| Production | Quality | Production | Quality | Total | ||
| Day Surgery | ||||||
| Propofol syringe, 20 ml | 28 | 1.00 | 0.11 | 32.76 | 5.79 | 38.55 |
| Propofol syringe, 50 ml | 28 | 1.00 | 0.11 | 32.76 | 5.79 | 38.55 |
| Remifentanil syringe, 20 ml | 20 | 1.20 | 0.13 | 39.32 | 6.95 | 46.26 |
| Remifentanil syringe, 50 ml | 20 | 1.20 | 0.13 | 39.32 | 6.95 | 46.26 |
| Dexamethasone syringe, 1 ml | 15 | 0.60 | 0.07 | 19.66 | 3.47 | 23.13 |
| Ketorolac syringe, 1 ml | 90 | 2.50 | 0.28 | 81.91 | 14.47 | 96.38 |
| * Atropine syringe, 1 ml | 15 | 0.83 | 0.09 | 27.30 | 4.82 | 32.13 |
| * Ephedrine syringe, 2 ml | 80 | 2.67 | 0.30 | 87.37 | 15.43 | 102.80 |
| * Suxamethonium syringe, 2 ml | 10 | 0.45 | 0.05 | 14.74 | 2.60 | 17.35 |
| Fentanyl syringe, 2 ml | 140 | 2.17 | 0.24 | 70.99 | 12.54 | 83.53 |
| Ondansetron syringe, 2 ml | 133 | 2.33 | 0.26 | 76.45 | 13.50 | 89.95 |
| Alfentanil syringe, 2 ml | 130 | 2.17 | 0.24 | 70.99 | 12.54 | 83.53 |
| Bupivacaine syringe, 2.5 mg/ml, 10 ml | 10 | 0.75 | 0.08 | 24.57 | 4.34 | 28.91 |
| Bupivacaine syringe, 2.5 mg/ml, 20 ml | 50 | 2.75 | 0.31 | 90.10 | 15.92 | 106.02 |
| Bupivacaine syringe, 5 mg/ml, 10 ml | 10 | 0.75 | 0.08 | 24.57 | 4.34 | 28.91 |
| Bupivacaine syringe, 5 mg/ml, 20 ml | 50 | 2.75 | 0.31 | 90.10 | 15.92 | 106.02 |
| Lidocaine-Epinephrine syringe, 10 mg/5 micrograms/ml, 10 ml | 10 | 0.75 | 0.08 | 24.57 | 4.34 | 28.91 |
| Bupivacaine-Epinephrine syringe, 2.5 mg+5 micrograms/ml, 20 ml | 50 | 2.75 | 0.31 | 90.10 | 15.92 | 106.02 |
| Bupivacaine-Epinephrine syringe, 5 mg+5 micrograms/ml, 20 ml | 50 | 2.75 | 0.31 | 90.10 | 15.92 | 106.02 |
| Endoscopy | ||||||
| RTU Midazolam syringe, 3 ml | 133 | 2.50 | 0.28 | 81.91 | 14.47 | 96.38 |
| Alfentanil syringe, 2 ml | 130 | 2.17 | 0.24 | 70.99 | 12.54 | 83.53 |
| Preparation times were obtained by observations in the hospital pharmacy | ||||||
| Average hourly staff cost was calculated using the following hourly costs: pharmacy production staff: €33, pharmacy quality staff: €52 | ||||||
Table 2: Observed preparation time in the hospital pharmacy and time costs per batch of medicine.
Handling costs (Ch) without RTU included the time that nurses used to prepare the medication trolley for the following day. Handling time for the RTU programme included time used by the clinical pharmacist to handle, un-pack the RTU deliveries and prepare the medicine box as well as staff time used at the hospital pharmacy logistic department for handling and shipping the RTU deliveries. An assessment of the average daily time use was made and was considered as a cost required for each department with the RTU programme.
Additional transport services (Ct) were required for the RTU-program due to the short shelf life of some of the prefilled syringes. It was assumed that with the RTU-program the day surgery department required an additional daily delivery from the hospital pharmacy. This was included as an average cost based on discussion with the local financial department.
Unit Costs
Hourly unit costs for staff were based on average gross salaries obtained from the hospital personnel system for each staff group at department level. The mean hourly salaries were uplifted with a factor 1.3, corresponding to the addition of 30% to cover days off work, meetings, and breaks. At departments without the RTU programme anaesthetists prepared some medicines (e.g. propofol, remifentanil and ephedrine) while the nurses assisting the surgeon prepared others (e.g. lidocaine-epinephrine, bupivacaine and bupivacaine-epinephrine). This implied different valuation of the preparation time dependent on the specific medicine. At the pharmacy syringes were produced in the production unit, and staff from the quality unit performing quality assurance. This also implied different cost of staff time in the production and quality assurance. The mean batch-cost for staff time in production and quality assurance was estimated in Table 3. The unit cost per syringe was calculated as the mean batchcost divided by the batch size. In the RTU scenario, all deliveries from the pharmacy were multiplied by factor 1.03 to account for a 3% overhead contribution.
| Without RTU | RTU | Cost difference per syringe | ||||||||
| Labor cost per syringe | Costs drug/syringe | Total costs per syringe | Labor cost per syringe | Additional production costs per syringe | Costs drug/syringe | Total costs per syringe | ||||
| Day Surgery | ||||||||||
| Propofol syringe, 20 ml | 0.58 | 1.04 | 1.62 | 1.38 | 1.31 | 1.07 | 3.76 | 2.14 | ||
| Propofol syringe, 50 ml | 0.58 | 2.50 | 3.07 | 1.38 | 1.31 | 2.57 | 5.26 | 2.19 | ||
| Remifentanil syringe, 20 ml | 0.96 | 1.04 | 2.00 | 2.31 | 1.63 | 1.07 | 5.02 | 3.02 | ||
| Remifentanil syringe, 50 ml | 0.96 | 2.51 | 3.47 | 2.31 | 1.82 | 2.58 | 6.72 | 3.25 | ||
| Dexamethasone syringe, 1 ml | 0.47 | 0.61 | 1.08 | 1.54 | 0.79 | 0.63 | 2.96 | 1.88 | ||
| Ketorolac syringe, 1 ml | 0.47 | 1.53 | 2.00 | 1.07 | 0.67 | 1.58 | 3.32 | 1.32 | ||
| * Atropine syringe, 1 ml | 0.47 | 1.04 | 1.51 | 2.14 | 0.52 | 1.07 | 3.74 | 2.23 | ||
| * Ephedrine syringe, 2 ml | 0.47 | 3.56 | 4.03 | 1.29 | 0.90 | 3.67 | 5.85 | 1.82 | ||
| * Suxamethonium syringe, 2 ml | 0.47 | 0.90 | 1.36 | 1.73 | 0.51 | 0.92 | 3.17 | 1.80 | ||
| Fentanyl syringe, 2 ml | 0.47 | 0.34 | 0.80 | 0.60 | 0.58 | 0.35 | 1.52 | 0.72 | ||
| Ondansetron syringe, 2 ml | 0.47 | 0.43 | 0.89 | 0.68 | 0.60 | 0.44 | 1.72 | 0.83 | ||
| Alfentanil syringe, 2 ml | 0.47 | 0.66 | 1.13 | 0.64 | 0.64 | 0.68 | 1.97 | 0.84 | ||
| Bupivacaine syringe, 2.5 mg/ml, 10 ml | 0.42 | 2.04 | 2.46 | 2.89 | 1.00 | 2.10 | 6.00 | 3.54 | ||
| Bupivacaine syringe, 2.5 mg/ml, 20 ml | 0.42 | 4.02 | 4.44 | 2.12 | 1.30 | 4.14 | 7.56 | 3.12 | ||
| Bupivacaine syringe, 5 mg/ml, 10 ml | 0.42 | 1.91 | 2.33 | 2.89 | 1.00 | 1.97 | 5.86 | 3.53 | ||
| Bupivacaine syringe, 5 mg/ml, 20 ml | 0.42 | 3.77 | 4.19 | 2.12 | 1.27 | 3.88 | 7.28 | 3.09 | ||
| Lidocaine-Epinephrine syringe, 10 mg/5 micrograms/ml, 10 ml | 0.42 | 1.09 | 1.51 | 2.89 | 1.22 | 1.12 | 5.23 | 3.72 | ||
| Bupivacaine-Epinephrine syringe, 2.5 mg+5 micrograms/ml, 20 ml | 0.42 | 4.64 | 5.06 | 2.12 | 1.35 | 4.78 | 8.25 | 3.19 | ||
| Bupivacaine-Epinephrine syringe, 5 mg+5 micrograms/ml, 20 ml | 0.42 | 4.75 | 5.16 | 2.12 | 1.37 | 4.89 | 8.37 | 3.21 | ||
| Endoscopy | ||||||||||
| RTU Midazolam syringe, 3 ml | 0.16 | 0.43 | 0.59 | 0.72 | 0.64 | 0.44 | 1.81 | 1.22 | ||
| Alfentanil syringe, 2 ml | 0.12 | 0.66 | 0.78 | 0.64 | 0.64 | 0.68 | 1.97 | 1.18 | ||
| Average hourly labor cost was obtained from the administrative systems: anesthetic staff: €32, endoscopy and surgical staff: €35, pharmacy production staff: €33, pharmacy quality staff: €52 Costs of drug/syringe were extracted from the hospital pharmacy administrative system Cost per syringe (RTU) includes a 3% overhead, which is added to all drugs prepared in the pharmacy |
||||||||||
Table 3: Calculated clinical costs per syringe with and without an RTU programme and calculated cost differences per syringe (2013-€).
The costs of medicines and syringes (Cd/s) were obtained from the price register at the hospital pharmacy and were reported as the purchase prices for the hospital pharmacy (excluding value added tax). This cost was lower than the market price because different suppliers offer various rebate agreements for the hospital pharmacy.
Additional production costs (Cap) included other consumables used by the pharmacy to produce the RTU syringes, which were not used by the clinical departments when preparing syringes (e.g. needles with special filter, pumps and storage bags). Additional production costs were calculated for each specific batch production in the pharmacy production unit. The additional production costs were divided by the number of syringes in the specific batch-production to get the production costs per syringe.
Sensitivity Analysis
Since each single cost item of the analysis potentially influenced the final cost estimates, different assumptions regarding perspectives and changes were identified and tested in sensitivity analyses. The total cost could potentially be influenced by the Discard rate (D), Preparation time (Pt) and Costs of medicine/syringe (Cd/s).
The following assumptions were tested in the sensitivity analyses:
1. Preparation time at the pharmacy production site was reduced by 30-50%. This assumed that, in the future, the optimizing/ automating production would further improve efficiency and thereby reduce the preparation time.
2. Discard rate was tested with a reduction of 70-90%: assuming that prolonged shelf life could almost eliminate discards. This would, however, be accompanied by increased costs of syringes, since more expensive syringes suitable for storage is a condition for prolonged shelf life. On this basis, a reduced discard rate was combined with an additional cost of medicine/syringes of €1.5 per syringe, which was the actual cost of these suitable syringes.
3. The best economic scenario was tested, assuming that all time used for preparation at the departments could be released for other tasks, while the preparation time in the pharmacy could be reduced by 50%, due to automated production. At the same time, the discard rate was assumed reduced by 90%, with no additional syringe costs. The best economic scenario was hypothetical and unlikely to be realized; however, adding information on minimum amount of expected additional costs.
Observations in the clinical setting prior to implementation revealed central processes expected to be affected by the RTU programme and, thus, relevant cost items to be included in the cost analysis. Using a micro-costing approach, each individual activity was measured and valued through several data collections and extractions. To perform the cost analysis, we used the cost model calibrated with relevant cost levels obtained for the specific local setting.
As a safety precaution, the discard rate for emergency medicines was high without RTU: 1.17 for ephedrine, 1.32 for suxamethonium and 1.62 for atropine. Implementation of the RTU programme resulted in an increased discard rate for suxamethonium and atropine to 2.31 and 3.47, respectively, whereas ephedrine showed a slightly decreased discard rate at 1.12. For other medicines, the discard rate increased in general, while the discard rate for local anaesthetics remained unchanged.
The costs of medicines, syringes, and production costs are presented in Table 3. The results showed that preparation costs increased considerably when syringes were prepared at the hospital pharmacy. These higher costs were caused by standard quality procedures in the pharmacy. Additional production costs were introduced by the hospital pharmacy in the formulation of RTU medicines. Such costs were not present without RTU. Increased time usage and additional production costs resulted in a cost per syringe increase of 50–250%, with an average per syringe cost increase of 120%.
The costs of the full RTU programme showed a total cost increase with the introduction of RTU delivery (Table 4).
| Without RTU | RTU | Cost difference (∆TC) | ||||||||
| Consumption | Costs per syringe | Total costs per drug | Consumption | Costs per syringe | Total costs per drug | ∆TCD = (TCD (RTU) + Ch + Ct) - TCD (without RTU) | ||||
| Day Surgery | 3 Months | 1 year | ||||||||
| Propofol syringe, 20 ml | 7 | 1.62 | 102 | 71 | 3.76 | 267 | 165 | 584 | ||
| Propofol syringe, 50 ml | 837 | 3.07 | 2,573 | 855 | 5.26 | 4,497 | 1,924 | 6,809 | ||
| Remifentanil syringe, 20 ml | 82 | 2.00 | 164 | 98 | 5.02 | 492 | 328 | 1,160 | ||
| Remifentanil syringe, 50 ml | 719 | 3.47 | 2,492 | 742 | 6.72 | 4,985 | 2,493 | 8,820 | ||
| Dexamethasone syringe, 1 ml | 83 | 1.08 | 89 | 90 | 2.96 | 267 | 177 | 627 | ||
| Ketorolac syringe, 1 ml | 498 | 2.00 | 996 | 540 | 3.32 | 1,793 | 797 | 2,821 | ||
| * Atropine syringe, 1 ml | 179 | 1.51 | 270 | 385 | 3.74 | 1,438 | 1,168 | 4,133 | ||
| * Ephedrine syringe, 2 ml | 501 | 4.03 | 2,016 | 479 | 5.85 | 2,803 | 787 | 2,784 | ||
| * Suxamethonium syringe, 2 ml | 142 | 1.36 | 194 | 250 | 3.17 | 792 | 598 | 2,116 | ||
| Fentanyl syringe, 2 ml | 1,930 | 0.80 | 1,551 | 1,980 | 1.52 | 3,011 | 1,459 | 5,164 | ||
| Ondansetron syringe, 2 ml | 721 | 0.89 | 645 | 790 | 1.72 | 1,359 | 714 | 2,527 | ||
| Alfentanil syringe, 2 ml | 121 | 1.13 | 137 | 220 | 1.97 | 433 | 296 | 1,049 | ||
| Bupivacaine syringe, 2.5 mg/ml, 10 ml | 25 | 2.46 | 61 | 25 | 6.00 | 150 | 88 | 313 | ||
| Bupivacaine syringe, 2.5 mg/ml, 20 ml | 350 | 4.44 | 1,552 | 350 | 7.56 | 2,645 | 1,092 | 3,866 | ||
| Bupivacaine syringe, 5 mg/ml, 10 ml | 55 | 2.33 | 128 | 55 | 5.86 | 323 | 194 | 688 | ||
| Bupivacaine syringe, 5 mg/ml, 20 ml | 120 | 4.19 | 503 | 120 | 7.28 | 873 | 370 | 1,311 | ||
| Lidocaine-Epinephrine syringe, 10 mg/5 µg /ml, 10 ml | 40 | 1.51 | 60 | 50 | 5.23 | 262 | 201 | 712 | ||
| Bupivacaine-Epinephrine syringe, 2.5 mg+5 µg/ml, 20 ml | 370 | 5.06 | 1,872 | 380 | 8.25 | 3,136 | 1,264 | 4,473 | ||
| Bupivacaine-Epinephrine syringe, 5 mg+5 µg /ml, 20 ml | 450 | 5.16 | 2,324 | 450 | 8.37 | 3,768 | 1,445 | 5,111 | ||
| Total syringes | 7,286 | 17,729 | 7,930 | 33,292 | 15,563 | 55,069 | ||||
| Handling costs (Ch) | 1211 | 4,600 | 3,389 | 11,992 | ||||||
| Transportation costs (Ct) | 0 | 963 | 963 | 3,409 | ||||||
| TC | 18,940 | 38,856 | 19,915 | 70,469 | ||||||
| Endoscopy | ||||||||||
| RTU Midazolam syringe, 3 ml | 1,543 | 0.59 | 905 | 1,640 | 1.81 | 2,967 | 2,062 | 7,298 | ||
| Alfentanil syringe, 2 ml | 2,147 | 0.78 | 1,684 | 2,190 | 1.97 | 4,310 | 2,626 | 9,292 | ||
| Total syringes | 3,690 | 2,589 | 3,830 | 7,277 | 4,688 | 16,590 | ||||
| Handling costs (Ch) | 0 | 1,220 | 1,220 | 4,316 | ||||||
| Transportation costs (Ct) | 0 | 0 | 0 | 0 | ||||||
| TC | 2,589 | 8,497 | 5,908 | 20,905 | ||||||
Table 4: Total costs per medicine without and with RTU, and quarterly and annually cost differences (2013-€).
The three-month increase from €18,940 without RTU delivery to €38,856 with RTU delivery for the day surgery department corresponded to an annual additional cost of RTU of €70,469 and, thereby, an increase by a factor of 2.05. For the endoscopy department, this additional cost amounts to €20,905, which is an increase by a factor of 3.28. These cost calculations clearly demonstrate a considerable budget impact of the RTU programme on both departments.
The cost analysis of the RTU programme was related to the productivity of the departments in order to calculate the incremental cost per operation, which would be relevant when applying the results to other settings with different productivity. The total costs per year (TC) of each alternative were divided by the annual number of operations/patients, which provided the average cost per operation. From this, the cost difference per operation or additional medication costs per operation using RTU syringe delivery can be evaluated. The annual production at the day surgery and endoscopy departments in 2013 was 6,225 surgeries and 8,664 endoscopies, respectively (Table 5). Relating the costs and cost difference to productivity showed that the RTU scenario resulted in an additional cost per operation of €11.32 at the day surgery department and €2.41 at the endoscopy department.
| Department | N operations | TC(without RTU)/year | TC( RTU)/year | ΔTC/year | TC(without RTU)/operation | TC( RTU)/operation | ΔTC/operation |
|---|---|---|---|---|---|---|---|
| Day surgery department | 6,225 | 67,020 | 137,489 | 70,469 | 10.77 | 22.09 | 11.32 |
| Endoscopy department | 8,664 | 9,161 | 30,066 | 20,905 | 1.06 | 3.47 | 2.41 |
| Data source: production data (2013) was extracted from the hospital administrative system | |||||||
Table 5: Annual costs and cost difference related to productivity of the departments (2013-€).
The results of the sensitivity analyses are presented in Table 6.
| Day surgery department | Endoscopy department | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TC | ∆TC range | Relative cost increase (TCRTU/TC without RTU) | TC | ∆TC range | Relative cost increase (TCRTU/ TC without RTU) | ||||||||||
| (per year) | (per year) | (per year) | (per year) | ||||||||||||
| Without RTU | 67,020 | 9,161 | |||||||||||||
| With RTU | 137,489 | 70,469 | 105% | 30,066 | 20,905 | 228% | |||||||||
| Scenario 1 | 50,983 | - | 41,813 | 76% | - | 62% | 18,150 | - | 16,313 | 198% | - | 178% | |||
| Scenario 2 | 107,789 | - | 104,816 | 161% | - | 156% | 42,722 | - | 42,369 | 466% | - | 463% | |||
| Best economic scenario | 35,896 | 54% | 15,641 | 171% | |||||||||||
| Scenario 1: Pharmaceutical production time reduction 30-50% | |||||||||||||||
| Scenario 2: Discard rate reduction 70-90% + additional syringe cost of 1.5ϵ Best economic scenario: pharmaceutical production time reduction 50%, discard rate reduction 90%, no additional syringe costs |
|||||||||||||||
Table 6: Results from the sensitivity analyses (2013-€).
Scenario 1 resulted in a considerable decrease in additional costs when calculating the intervals of potential reduced production time in the pharmacy.
Scenario 2 revealed an increased cost difference, indicating that a high discard rate, due to cheap syringes and, thereby, short shelf life, was less expensive than a low discard rate and syringes aimed for storage. A relatively great impact was observed in the endoscopy department, which is due to their large consumption of small syringes.
The best economic scenario showed that implementation of RTU syringe delivery, even in an optimal setting, was associated with higher overall costs. In this scenario, the annual cost increased by 54% in the day surgery department and by 171% in the endoscopy department. This scenario is not likely achievable but is relevant to illustrate the best possible RTU scenario.
Comparing the results of the sensitivity analysis highlights the specific importance of the cost item Preparation time, since this item showed the greatest impact of the final results, whereas the effect of the other cost items were less important. These results demonstrate that the largest possible cost reduction can be gained by focusing on development of the production process.
In this study, we identified relevant cost items and developed a cost model that was used to assess the costs of different ways of medicine delivery. The results showed that the RTU syringe delivery programme imposed additional costs and that the relative cost increase was fairly high. The increase was partly due to the fact that the applied medicines have become very cheap in their current formulation without RTU.
Implementation of RTU was found to be sensitive to preparation time, indicating potential cost savings with automated production and economies of scale. The sensitivity to preparation time underscores the importance of the validity and accuracy of preparation time estimates. One of the strengths in this study was the careful measurement of preparation time, which followed a protocol and aimed to ensure that variations among different staff and situations were collected in order to calculate a valid and clinical relevant estimate of mean time.
Strength of the study was the establishment of the cost model, which allowed for evaluation of changes to selected cost items to reveal the impact on overall cost. Although major changes in the cost structure were tested, all analyses showed a cost increase, suggesting it is unlikely for an RTU programme to save costs in similar settings. The specific budget impact in other settings will, however, be dependent on local legislation, different settlement models and budget structure used within the given health care system.
The additional costs of the RTU programme were explained by the difference in guidelines for preparation of medication in the clinical departments and in the hospital pharmacy. The hospital pharmacy prepares medicine under aseptic conditions and must comply with national guidelines for sterile processing, and is subject to surveillance by the Danish Health Authority. This sterile processing is more time consuming than preparing syringes in the operating rooms, which may not meet these demands of sterility. Moreover, the production in the pharmacy introduces additional costs because additional equipment for aseptic and semi-automated production will be needed. However, these additional steps in the production process of syringes clearly imply that the quality of prefilled syringes increases when prepared at the pharmacy. Increased quality of the product could potentially result in reduced risk of contamination of the medicine, and thereby potentially lower the risk of post-operative complications for the patient [3].
In this study, the prefilled syringes were produced by the hospital pharmacy, since industrially produced prefilled syringes were unavailable at the Danish market. Industrial production could imply economies of scale with lower costs per syringe as a result. This could limit the generalizability of the study results to some countries where the supply is different. However, transparent preparation of the cost calculations used in this study allows for direct comparison of syringe costs (Table 2).
Bellefleur et al. concluded that the prefilled ephedrine was cost-effective, due to reduced consumption and discard rates [16]. These results were supported by the findings of Crégut-Corbaton et al. [17]. This present study also showed a slightly reduced discard rate of ephedrine; however, this reduction did not result in ephedrine being cost-effective. This contrasts with the French findings and is likely related to the Danish procedures and medication handling processes. The specific procedures and use of medication were also found to be fundamental in analysing the cost impact of prefilled syringes in a survey by Vipond and de Mello, who, like this present study, found that prefilled syringes were associated with increased costs [19].
The study of Webster et al. [18], found the new drug administration system, including prefilled syringes, to be associated with a significant cost increase of €23 per anaesthetic [18]. In this study, we found an increase of €11.32 per anaesthetic. This difference in results could be explained by the setting, since the day surgery department performs uncomplicated surgery, anaesthetics are short and most likely resulting in a smaller consumption of syringes. However, both studies show that prefilled syringes are associated with increased costs.
The discard rate of the RTU programme was very high (Supplementary File 2), which was caused by the combination of very short shelf life and unpredictable use of the different medicines in the department. Production of prefilled syringes aimed for storage would result in prolonged shelf life and may decrease the discard rate. This scenario was tested in the sensitivity analysis, and a major increase of additional costs made it economically preferable to choose disposable syringes, short shelf life, and high discard rate. These findings contribute to the discussion of strategy within the hospital pharmacies when organizing RTU syringe delivery demanded by hospitals that have a tight budget and high expectations regarding quality of the product.
This study adds information about cost impact of prefilled syringes. However, lower risk of post-operative complications, reduced risk of errors found with RTU-delivery [7,8,18] and thereby less adverse drug events (ADE) are all safety issues potentially resulting in cost-savings, which must be related to the incremental cost increase. A study by Lahue et al. [20] estimated the costs of preventable ADEs associated with injectable medications. They found the average extra cost per hospital to be $ 600,000 (~€ 540,000). Of course, these potential cost savings depend on the activity levels of the hospital but imply that the incremental cost introduced by RTU-delivery could be partly off-set by better safety effects. To investigate this further relevant future research could include full health economic evaluations, where the incremental cost analysis is related to the incremental effect in terms of avoided medication errors. This could support decision makers in determining whether or not prefilled syringes or full RTU programmes represent “good value for money”.
This study identified cost items relevant for incremental cost analyses of the implementation of RTU syringe delivery programmes.
The cost analysis showed that the RTU programme resulted in additional costs to both departments, and showed that preparation time had the greatest impact on incremental cost and represents the largest challenge for the cost of implementing future programs with prefilled syringes.
The additional costs of prefilled syringes might be offset by improvements in patient safety; however stronger evidence for patient safety might be required.
This study could not have been performed without the intensive work of the project group, which has participated in the development of the RTU programme which included both clinical staff and staff from most departments of the Hospital Pharmacy.
We thank the supervisory group within the Hospital Pharmacy that has followed and supported the development of the RTU programme and the study.
The Steering committee and its members of hospital management at different regional hospitals have supported the study and made proposals for a change of regional guidelines, supporting the standardized use of labels in anaesthetic departments. This decision was fundamental in implementing and testing the programme of study.
Michael Wulff Risør is thanked for his support and discussions regarding the content of the study and for his critical revision of the manuscript.