ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
M. Mahadevan 2, K. Ramachandran1
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Single crystals of potassium para-nitrophenolate were successfully grown by the slow evaporation method with dimension of 25x10x7 mm3. Proton NMR spectrum was recorded to elucidate the molecular structure. Fourier transform infrared (FTIR) FT-Raman spectral studies have been performed to identify the functional groups. Structural analyses were carried out by powder x-ray diffraction pattern. Thermo gravimetric analysis (TGA) and differential thermal analysis (DTA) were used to study its thermal properties. Powder test with Nd: YAG laser radiation shows second harmonic generation. The optical transmittance window and the lower cut off wavelength of the grown crystal have been identified by UV–vis–NIR spectrum. The luminescence property has been studied by photoluminescence spectrum. The Micro hardness test was carried out and the load dependent hardness was measured. The observed optical properties have confirmed that the grown crystals are useful for linear and nonlinear optical applications
Keywords |
| Crystal growth, Proton NMR, Thermal analysis, nonlinear optical material. |
INTRODUCTION |
| In recent years, an intense effort has been focused worldwide on the design and development of highly efficient organic and semi organic nonlinear optical (NLO) materials [1-3]. The interesting nonlinear optical effects extended to optical amplifiers, optical parametric oscillators, Q-switched intra cavity second harmonic devices, high optical damage threshold and other electro-optical applications lead to the extensive research in organic functional group materials which becomes an expanding area. The organic ionic crystals exhibit the highest figure of merit for second harmonic generation, two photon absorption (TPA) processes and UV tuneable laser applications among the known organic and inorganic NLO materials, [4-6]. However, the absorption wavelength range of such organic materials, if they have a high SHG efficiency, extends to visible region and their crystals show yellow or orange colour, which makes them useless to wavelength conversion of semiconductor lasers. Semi organic nonlinear materials have the advantage over the problem said above and they are cheaper and can be synthesized by reasonably simple chemical methods. Many applications of nonlinear optics require single crystals in bulk form with high perfection. Para nitro phenol was identified as potential organic material which gives variety of derivatives with alkali metal hydroxides. In spite of its difficulty in growing optically clear bulk organic as well as semiorganic NLO crystals from aqueous solution for photonic device applications, several attempts are made to grow derivatives of p-nitro phenol from aqueous solution by slow evaporation method [7-13]. Potassium p-nitrophenolate dihydrate (NPK) is a recently identified potential NLO active semiorganic crystal possessing a large value of hyperpolarizabilities and belongs to acentric crystal classes [14]. Single crystals of potassium paranitrophenolate dihydrate (NO2-C6H4-OK. 2H2O) have been grown and some new bonding properties have been reported by Boaz et al [15]. Optical and spectroscopy studies of potassium p-nitrophenoplate dihydrate crystal for frequency doubling applications have been reported by Jose et al [16]. However, the very preliminary investigation for its structure like powder x-ray diffraction studies, thermal analyses and hardness of the NPK.2H2O crystals were left unstudied. Hence, to fill the gaps in the characterization of the title compound the crystals have been grown and the properties have been studied. In the present investigation, the growth aspects of NPK.2H2O is studied under the isothermal solvent evaporation technique and the grown crystals are characterized by powder crystal X-ray diffraction, Fourier transform infrared (FTIR) and UV visible spectral, thermal, micro hardness and PL Studies . The NLO activity in the crystal is confirmed by the Kurtz powder test and found to be 1.5 times that of KDP. |
II. EXPERIMENTAL PROCEDURES |
| The title compound was synthesized by taking equimolar ratio of paranitrophenol (98% Pure) and potassium hydroxide (Merck Product) and mixed thoroughly in a container using a magnetic stirrer. The obtained saturated solution was further purified and allowed to evaporate at higher temperature which yields powder form of the synthesized potassium p-nitrophenolate dihydrate. Synthesized material was purified by repeated recrystallization process. To realize the practical applications, good quality single crystals of reasonable size are essential. Single crystals were grown at room temperature (30ïÃâðC) in the solution and the crystals were carefully harvested from the solutions after 30 days. The saturated solution obtained according to the solubility data was kept in a beaker and allowed to evaporate continuously at constant temperature. Good quality seed crystal obtained from spontaneous nucleation is suspended in the saturated solution. By slow evaporation solution method, NPK.2H2O single crystals have grown with the size is 25x10x7 mm3 and shown in Fig. 1. The grown crystals has larger dimension than the previously reported ones with the shorter growth period [14-16]. The grown crystals were subjected to powdered X-ray diffraction studies by using Xpert Pro diffraction system for the structural confirmation. The Proton NMR spectrum of the sample was recorded using an AMX 400 MHz spectrometer in DMSO-d6 Perkin Elmer. The powder samples were mixed separately with KBr in 1:20 weight ratio and made as a pellet to obtain the Fourier transform infrared spectrum of the grown crystal by using Perkin Elmer Spectrometer in order to find the presence of various functional groups. FT-Raman spectrum has been recorded by using Bruker RFS 27 model spectrometer for the confirmation of functional groups. Thermo-gravimetric (TG) and Differential Thermal Analysis (DTA) for the crystal samples were carried out in nitrogen atmosphere by a Perkin Elmer Thermal Analyzer to study the thermal properties of the as-grown crystal. Linear optical properties of the crystals were studied by UV-Vis Spectrophotometer and nonlinear optical properties were tested by Kurtz Perry powder technique [17].The photoluminescence measurements were carried out by Perkin Elmer LS 55 Luminescence spectrometer using 520 nm as excitation wavelength. |
III. RESULT AND DISCUSSION |
X-RAY DIFFRACTION ANALYSIS |
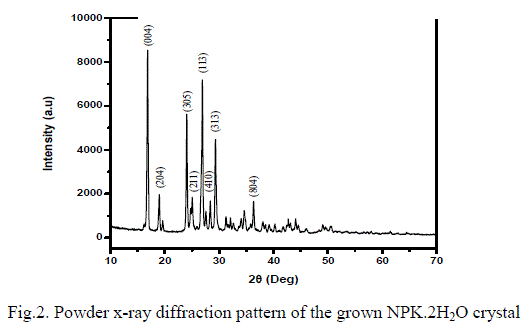
| From single crystal x-ray diffraction analysis it is found and reported that the crystal has monoclinic system with the lattice parameters of a= 22.098(2) Å, b= 3.7911(3) Å, c= 21.391(3) Å and β= 121.513Ãâ¹ÃÅ¡[14]. Then the grown crystals were made as fine powder and subjected to powder x-ray diffraction analyses. The data have been collected at 298 K between 10 and 80ïÃâð of diffraction angles (2ïÃÂñ) with the source wave length of 1.5460 Å. The step size of 2θ and the scan step time were fixed as 0.017ïÃâð and 10.3254 seconds respectively. The diffraction pattern contains various reflections corresponding to various crystallographic planes. The sharp peeks of the pattern have been observed due to the good quality of crystalline nature. From the lattice parameters and d spacing obtained by this experiment the miller indices were calculated and the powder XRD peaks were indexed. The indexed powder x-ray diffraction (PXRD) pattern of NPK.2H2O is shown in Fig. 2. |
PROTON NMR SPECTRAL ANALYSIS |
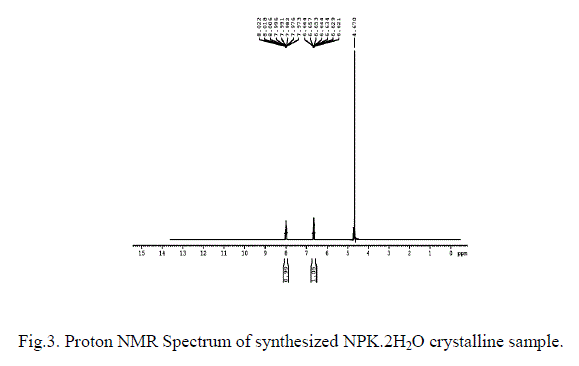
| Proton NMR spectral analysis has been carried out in order to confirm the structure of the synthesized material. The recorded proton NMR spectrum is shown in Fig. 3. In addition to the peek for the solvent deuterium oxide at 4.67 ppm two signals have been observed. The ortho hydrogen in the aromatic structure can see each other as aligned (parallel) or opposed (anti parallel) and usually come to resonance twice [17]. But, in this study the signals at 6.4 due to the ortho hydrogen of benzene ring and at 7.9 is due to the meta hydrogen of benzene ring. The multiple splitting is due to the functional groups present in the para position. Absence of any other peaks infers that the material has been synthesized as a pure NPK. |
FTIR AND FT-RAMAN SPECTRAL ANALYSIS |
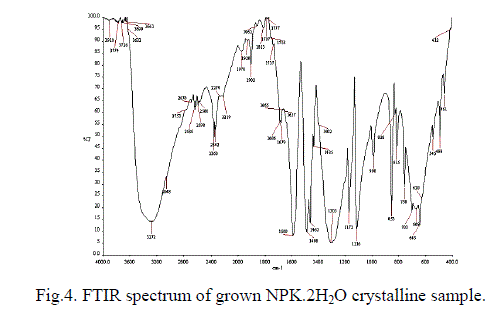
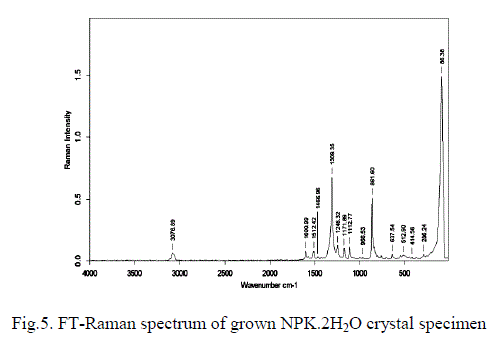
| For the purpose of analysing the presence of various functional groups in the grown NPK crystals the Fourier transform infrared spectra have been recorded in the frequency range between 400 and 4000 cm-1. The recorded FTIR spectra are shown in Fig. 4. The spectrum is found to be complex, due to the presence of various modes of vibrations. The symmetric and asymmetric stretching vibrations due to -OH of lattice water have been observed in the high frequency region near 3272 cm-1 as a broad band. Generally these modes of vibration have not been resolved clearly which confirm the presence of hydrogen bonded lattice water in the material. The bending vibration of H-OH of water molecule has been observed at 1665 cm-1. The sharp peak at 1116 cm-1 is due to C-O stretching vibration mode which is observed in the spectrum. The para substitution usually gives its vibration around 850 cm-1 is observed at 853 cm-1. The para substitution NO2 gives its symmetric stretching vibration from 1300 to 1310 cm-1 is present in the spectrum and the asymmetric stretching vibration is observed at 1589 cm-1. The vibration observed in the range of 600 to 700 cm-1 is due to various ring vibrations [16]. Bending vibration of the ring C-H is observed at 1488 cm-1. On the other hand, FT-Raman spectrum has also been recorded to confirm the functional groups and is shown in Fig. 5. Generally the unresolved peaks in the FTIR spectrum will be clearly resolved in FT-Raman Spectrum. In this investigation the vibration at 3048 and 1665 cm-1 in the FTIR spectrum due to H-OH stretching of water molecules is not clearly resolved. The same vibrations are observed clearly in the FT-Raman spectrum at 3077 and 1601 cm-1 respectively. In similar manner, the symmetric stretching of Para nitro group has been clearly observed at 1309 cm-1 in Raman spectrum rather than FTIR spectrum. Bending vibration of C-H present in the benzene ring has been observed at 1172, 1113, 862 cm-1 in Raman, but in FTIR spectrum it has been observed only at 1116 cm-1. |
THERMAL ANALYSIS |
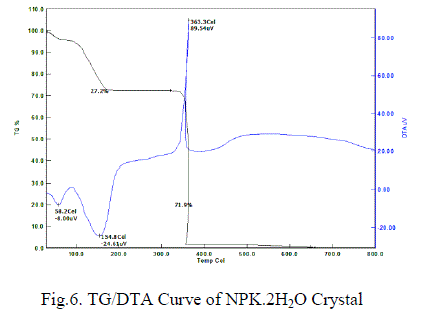
| Thermal analyses have been performed on the samples of grown crystal powder to study the thermal stability and melting point as they provide good thermal stability of the material for fabrication where a considerable amount of heat is generated during the cutting process. The thermo gravimetric analysis (TGA) of the NPK crystal has been carried out between room temperature (28ïÃâðC) and 800ïÃâðC at a heating rate of 10ïÃâðC per min. The experiment has been performed in nitrogen atmosphere and the TG & DTA plots are as shown in Fig. 6. The weight loss below 154ïÃâðC has been occurred in two stages in the powder samples of grown crystal which is assigned to loss of water in the TGA Curve. From the DTA curve these two stages have been observed at 58 C and 155 C which are due to weakly entrapped moisture and the lattice water respectively. A sharp peek around 363ïÃâðC in DTA curve with the sharp weight loss shown in TGA curves ensure that the simultaneous melting and decomposition of the crystal sample. The sharp melting point ensures that the grown crystals are of high purity. However the application of this material is restricted to very low temperature as the material loses it’s around 55ïÃâð C. This could be the probable reason for the restricted number literature on this material. |
VICKER’S MICRO HARDNESS STUDY |
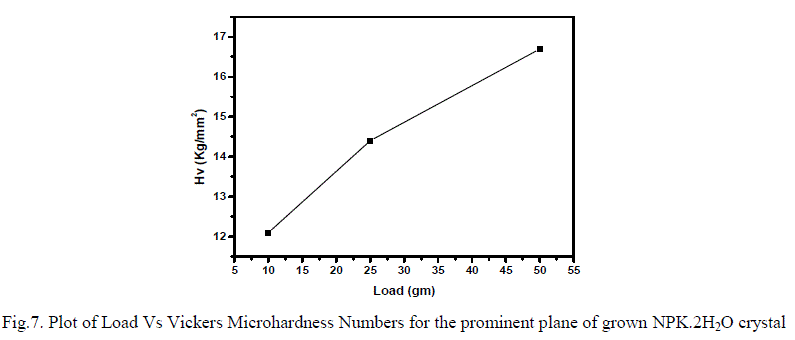
| Hardness of a crystal plays a key role in the device fabrication. It is a measure of a material's resistance to localized plastic deformation. The Vickers’s hardness number of the grown crystal has been calculated using the relationship Hv = 1.8544 P/d2 where, Hv is the Vickers’s micro hardness number, P is the applied load in kg and d is the average diagonal length of the impression in mm. Figure7 shows the variation of hardness number with applied load. Before indentations, these crystals have been lapped carefully and washed to avoid surface defects, which may influence the hardness values. The indentations were made on the prominent plane of the grown crystal for the loads varying from 10 to 50g with a time of 10s. The distance between two indentation points was maintained to be more than three times the diagonal length, in order to avoid any mutual interference of indentations. It is observed that the hardness value is increased as the load increased up to 50 gm of applied load for the prominent plane of the crystal because these loads are insufficient to soften the bonding in the molecules. Cracks were formed above 50g load due to release of internal stress generated locally by indentation. |
LINEAR AND NONLINEAR OPTICAL PROPERTIES STUDIES |
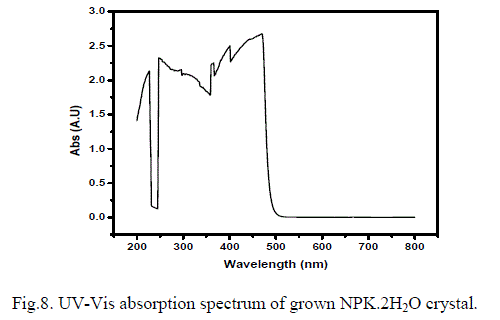
| It is essential to have fair optical transparency in an NLO crystal in the green visible region. Optical absorption spectrum for the grown crystal has been recorded in the range between 200 to 800 nm and is shown in Fig. 8. The grown crystal has UV cut off below 500 nm and above which the grown crystal is transparent in the entire visible range of the spectrum which has good agreement with the literature. The cut-off wavelength in the present investigation has quite reasonable agreement with the literature [14-16]. On the other hand the crystal grown in the present investigation has much lesser absorption to the previous ones which is most desirable for an efficient NLO material. This enhanced linear optical property may due to the higher order purity of the raw materials used which results in high purity of the grown crystals. So, the crystal in the present investigation has enhanced linear optical property and it is useful for optoelectronic applications and the second harmonic generation (frequency conversion) from the Nd: YAG Laser. The SHG efficiency of NPK crystalline samples have been found by Kurtz and Perry powder technique. The powder samples prepared from the grown crystals have been subjected to this test. The second harmonic output has been generated by irradiating the powder samples by a pulsed laser beam of fundamental wavelength 1064 nm, 8 ns pulse widths, with 10 Hz pulse rate was made to fall normally on the sample cell and output intensity of the SHG signal is measured with the photomultiplier tube. The energy (frequency) conversion capability is confirmed by the emission of green light from the powder sample of the grown crystal. KDP sample has been used as the reference material and output power intensity of the samples has been measured to have 1.52 times that of KDP. |
PHOTOLUMINESCENCE (PL) ANALYSIS |
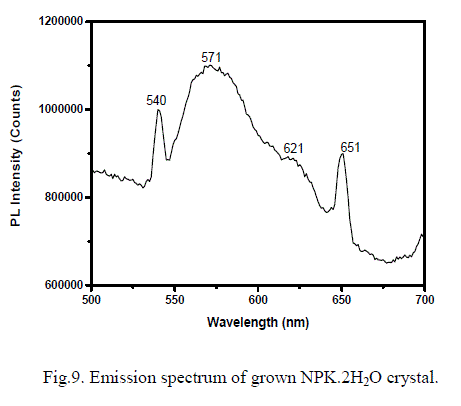
| Aromatic compounds or the molecules with multiple conjugated double bonds are expected to give high degree of resonance stability and can be expected strong fluorescence. Since the title compound contains aromatic structure, the emission spectrum has been recorded at room temperature by exiting the molecules with wavelength of 485 nm. The emission spectrum is shown in Fig. 9. Strong emission from green to red is observed with three peaks at 540, 571 and 651 nm for this excitation. This property of having strong emission in this range may lead to potential application of this material in optoelectronic devices [16]. |
IV. EXPERIMENTAL RESULTS |
 |
 |
 |
 |
 |
 |
 |
 |
 |
V. CONCLUSION |
| Optical quality single crystals of NPK.2H2O were grown using solution growth method by slow evaporation of solvent technique. Structural analyses were carried out by powder x-ray diffraction, NMR methods. The functional group was confirmed by FTIR and FT-Raman spectral analyses. The thermal behaviour of the grown crystals was studied by using TG-DTA curves. The hardness of the crystals has been determined by Vickers micro hardness method. From the optical absorbance spectra, it is observed that the NPK.2H2O crystal grown in the present investigation has a fairly wide transparency range and lesser absorbance than the previously reported crystals. Powder test with Nd:YAG laser radiation shows second harmonic generation and it is 1.5 times that of KDP. Thus, NPK.2H2O seems to be a promising material for NLO application. |
VI. ACKNOWLEDGEMENT |
| The Authors thank Sakthi Dr. B. SenthilKumar, Correspondent, Adhiparasakthi Engineering College, and Melmaruvathur, India for his constant motivation and support. |
References |
|