ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Ajeet Kumar Srivastava1, Pushpa Agrawal2, Abdul Rahiman3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Lignocellulose is a generic term for describing the main constituents in most plants, namely cellulose, hemicelluloses, and lignin. Lignocellulose is a complex matrix, comprising many different polysaccharides, phenolic polymers and proteins. Cellulose, the major component of cell walls of land plants, is a glucan polysaccharide containing large reservoirs of energy that provide real potential for conversion into biofuels. Lignocellulosic biomass consists of a variety of materials with distinctive physical and chemical characteristics. The conversion of Lignocellulosic biomass to ethanol involves pretreatment followed by polysaccharide hydrolysis to simple sugars followed by sugar fermentation to ethanol. The presence of lignin in cell walls negatively impacts these conversion steps. Sodium hydroxide treatment is one of the highly effective lignin removal methods due to their strong alkanity. The chemical pretreatments of rice husk were carried out with sodium hydroxide and sodium chlorite concentration (1- 5%) with the best results at 5% for both the solutions. This greatly enhanced its susceptibility to enzymatic hydrolysis at 30 °C. The chemical pretreatments caused deep deacetylation and milder delignification of rice husk and did not cause an apparent loss of cellulose. In addition fungal treatment (Trichoderma reesei) of pretreated samples has been applied to improve the conversion of cellulosic material in sugar. The method of alkali pretreatment and subsequent fungal treatment (in different concentrations) results the highest conversion of lignocelluloses in rice husk to sugar and consequently, highest ethanol yields were 250 mg/gram dry biomass after 6 days fermentation with Saccharomyces cerevisiae.
Keywords |
| Delignification, Bioethanol, Lignocellulosic biomass, Sodium hydroxide, sodium chlorite, Fungal treatment, Fermentation. |
INTRODUCTION |
| Ethanol productions from cellulosic materials offer a solution to some of the recent environmental, economic, and energy problems facing worldwide. Nationally, energy costs are on the rise and forecasts of petroleum supply disruptions are once again making news. People are not immune to these events & feel the impact of rising energy prices every time they purchase gasoline, diesel or other petroleum products. Cereal straw, one of the most abundant renewable lignocellulose resources which possess valuable components, has gradually become the research hot spot as a promising substitute for both the fossil fuel resource & petroleum based industry with the increasing calling for biofuel and green chemistry. Lignocelluloses are mainly comprised of cellulose, hemicellulose, and lignin[1],[2]. Lignocellulosic biomass is the largest source of hexose and pentose sugars, which can be used for the production of bioethanol[3]. Unlike first-generation biomass, in second-generation lignocellulosic substrates cellulose in the cell wall is encased within hemicellulose and lignin matrix, and thus accessibility of cellulose is a major problem in bioethanol production from such sources. Thus, the cost of bio-fuel production is high due to intensive labor and increased processing steps. These economic and technical obstacles must be overcome for efficient and cost effective biological conversion of lignocellulosic biomass into biofuels. The purpose of alkaline hydrolysis is one of the critical method used to pretreat the plant biomass, however the effect of alkaline pretreatment method depends on the lignin content of the materials [4][5][6][7][8][9][10]. The mechanism of alkaline hydrolysis is believed to be saponification of intermolecular ester bonds cross-linking xylan hemicelluloses and other components, for example, lignin and other hemicellulose. NaOH treatment of lignocellulosic materials caused swelling of lignocellulosic materials, leading to an increase in internal surface area, a decrease in the degree of polymerization, and crystallinity, separation of structural linkages between lignin and carbohydrates, and disruption of the lignin structure is commonly reported [6][11] The chlorite oxidation and wet oxidation are also used as promising oxidative delignifying pretreatments. [12] used wet oxidation and alkaline hydrolysis of wheat straw (20 g straw/l, 170 °C, 5–10 min), and achieved 85% conversion yield of cellulose to glucose. Whereas, the sodium chlorite treatment yielded approximately 90 % delignification in woody material (Prosopis juliflora; Lantana camara) [13][14]. |
| The bioconversion process is mainly consist of 3 major steps i.e, pretreatment, hydrolysis, and fermentation. The purpose of the pretreatment is to remove lignin and hemicellulose fraction, reduce cellulose crystallinity and increase the porosity of the materials. The pretreated material is subjected to the enzymatic hydrolysis and the resultant hydrolysates are fermented to ethanol using fermenting microbes[15]. Cellulose can be effectively hydrolyzed and depolymerized into fermentable sugars by the enzyme cellulase[16],[17],[18],[19]. Currently most commercial cellulases are produced from Trichoderma reesei [20], [21] usually used to describe a mixture of cellulolytic enzymes action is required for effective breakdown of substrate to its monomeric units. The action of cellulases involves the concerted action of (i) endoglucanases (endo-1, 4- β -glucanases, EGs) can hydrolyze internal bonds preferably in cellulose amorphous regions releasing new terminal ends, which randomly attacks the internal, β1,4-linkages (ii) cellobiohydrolase, (exo-1, 4 β --glucanases, CBHs) act on the existing or endoglucanase generated chain ends (iii) β 3-glucosidase, which hydrolyzes cellobiose to glucose. |
OBJECTIVES |
| i. To find out alternative sources of bioenergy as the prime source of biofuels |
| ii. To use Potential availability of some agricultural residues in India like rice husk as raw materials. |
| iii. To achieve high yield of sugar by different pretreatment methods like alkali and chlorite treatments |
| iv. To achieve high yields of fermentable sugar from lignocellulosic components through hydrolysis using effective microorganism |
| v. To determine the effect of sugar yield using microorganisms like Trichoderma reesei |
| vi. To achieve high yields of ethanol at economic level using fungi S. cerevisiae. |
MATERIAL AND METHODS |
| Raw materials |
| Rice husk from local mill powdered and sieved into a 1 mm seiver. Powder of the raw material was used as carbon source. |
| Microorganisms |
| Trichoderma reesei (MTCC-4876) were obtained from, MTCC Chandigarh. The fungi produces cellulolytic enzymes that converted carbohydrate polymers into fermentable sugars Later Saccharomyces cerevisiae was inoculated to utilize reducing sugars to ethanol. |
| Inoculum-preparation |
| Fungal cultures were inoculated onto PDA medium in the Petri plate. Spores scraped out from 7 days old slants were dispersed in desired quantity of sterile distilled water containing 0.1% Tween 80 and vortexed. Spore count was measured with haemocytometer and adjusted to 2x106 spores/ml by adjustment of optical density. |
| Culture-medium |
| 1000 ml of Mandels medium was prepared by adding (in gms) Urea 0.3, (NH4)2SO4 1.4, KH2PO4 2.0, CaCl2.2H2O 0.4, MgSO4.7H2O 0.15, bactopeptone 1.0, and yeast extract 0.25. Trace elements were also added (in mg), FeSO4.7H2O 0.15, MnSO4.H2O 1.6, ZnSO4..7H2O 1.4, CoCl2 2.0. pH was adjusted to 5.5-6.0 before sterilization[22]. |
| Culture-conditions |
| 5g / 100 ml (Mandel’s medium) of each substrate was taken in conical flask (250 ml).The conical flasks were plugged with cotton and sterilized at 15lbs per sq.inch for 30 minutes. The flasks were inoculated with the fungul strains in their different concentrations (ml). These flasks were incubated at room temperature for 6 days on an orbital shaker. After six days mycelium was separated by filtration through Whatman filter paper. The filtrate was used for further studies[23]. |
| Pretreatment-Strategies |
| Chemical-pretreatments |
| The 5 grams powdered rice husk was taken for further process. The dried substrate was chemically treated with sodium hydroxide solution concentration (1-5%) and sodium chlorite (5%) and after that washed several times with deionised water and then dried with hot air oven for 24 hrs at 70Ãâ¹ÃÅ¡C .Later on dry fibrous cake was weighed which further processed for microbial saccharification |
| Biological-pretreatments The biological pretreatment was carried out with fungal treatment, Trichoderma reesei. |
| Fermentation of Hydrolysates to Ethanol |
| After saccharification, Saccharomyces cerevisiae was inoculated for utilization of reducing sugars to ethanol. The microorganisms strain was grown in SDDL broth (glucose 20.0, yeast extract, 5.0 g L-1) at 30ºC for 48 hrs. The number of viable cells 109 (cfu/ml) was determined by the agar plate method using Schreder agar (MgSO4.7H2O 0.5, (NH4)2SO4 1.0, KH2PO4 1.0, yeast extract 1.0, sucrose 20, agar 15 g L-1) incubated for 24 hrs at 30ºC. |
| Analytical Methods |
| The carbohydrate content of pretreated raw materials in the culture broth was measured by Anthrone method [24]. The amount of reducing sugars in pretreated raw materials in the culture broth were determined by dinitrosalicylic acid (DNS) method [25] with glucose as standard. The amount of Non-reducing sugar present in the sample was calculated by subtracting Reducing sugars from Total soluble sugars. Soluble protein was analyzed by the method of Lowry et al. [26] The estimation of pentose sugars was carried out using the fuming Ferric chloride (Iron solution) and orcinol reagent, as described by Standing et al [27]. Determination of ethanol content was done by spectrophotometric method [28]. Cellulase enzyme production was studied by FPU assay [29]. |
RESULTS AND DISCUSSION |
Pretreatment |
| Compositional analysis of lignocellulosic substrate |
| Cellulose, the dominant structural polysaccharide of plant cell walls, was ranging from 35 to 50%, followed by hemicelluloses (20-35%) and lignin (10-25%). |
| Pretreatment strategies (Chemical and biological pretreatments) |
| The effect of NaOH a n d Na-Chlorite treatments on the lignocellulosic substrate (rice husk) was also studied for varied concentrations and pretreatment time. The maximum results were obtained with 5% NaOH and 5% with Na |
| Chlorite as shown in figures 1 and 2. |
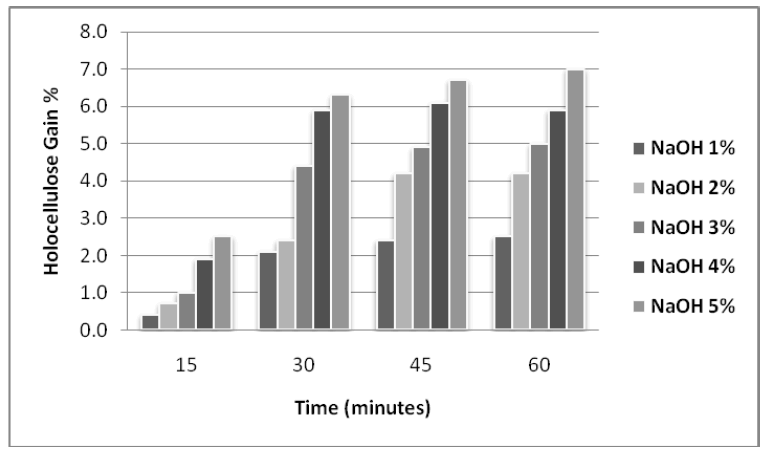 |
| Figure 1: Effect of different concentration of alkali on holocellulose enrichment in Rice Husk at different time intervals |
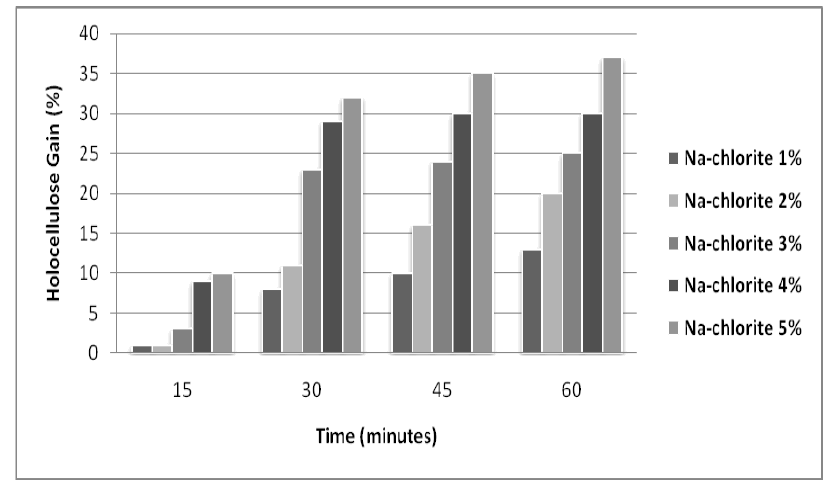 |
| Figure 2: Effect of different concentration of Na-chlorite on holocellulose enrichment in Rice Husk at different time intervals |
| Saccharification |
| Effect of Commercial Cellulase Enzyme on the Hydrolysis of Delignified Substrate |
| The substrates were treated with FPase dosages for saccharification. The optimum sugar release (740.35 ± mg/gds) was with rice husk substrate. |
| Microbial Saccharification of Delignified Substrate |
| One Factor at a Time approach (figure 3) of microbial saccharification (using fungi) of delignified substrate was studied. It was revealed that the optimum sugar released (127.10 ± 4 .21 mg/g) was obtained on the seventh day of saccharification. |
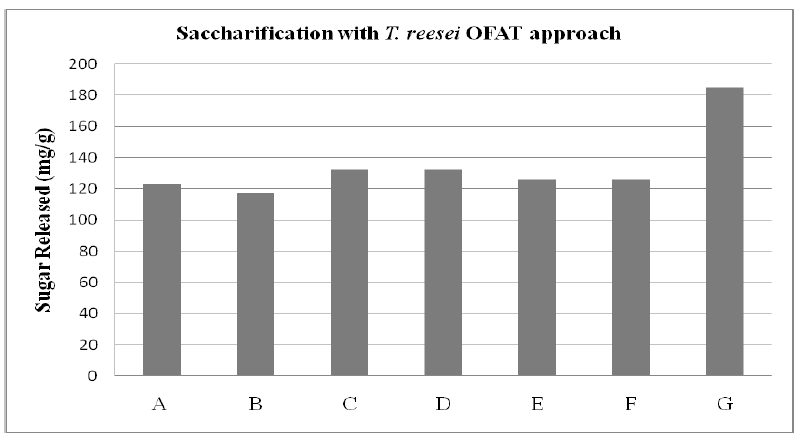 |
| Figure 3: Rice husk saccharification where A is Time (7 days), B is Substrate (5 %), C is Microorganism Concentration (5 ml), D is Temperature (30oC), E is Agitation Rate (200 rpm), F is pH (5.5) and G Tween 80 (1% v/v) |
| Fermentation of Detoxified Acid Hydrolysate |
| Ethanol production increased with increase in the incubation time till 7 days of incubation. However, the maximum ethanol obtained was 3.20 ± 0.36 g/l with and ethanol yield (0.27 g/g total sugar) (figure 4). |
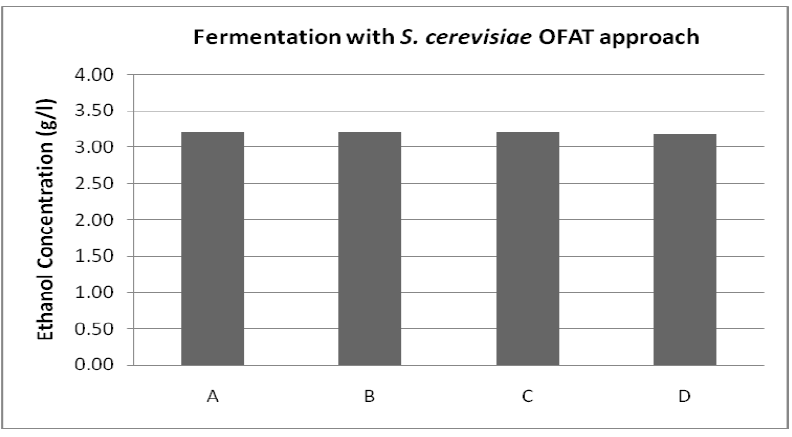 |
| Figure 4: Fermentation with S. cerevisiae where A is Time (6 days), B is pH (6.0), C is Temperature (30OC), D is Concentration of Soybean meal (1.20 % (v/v)) |
| The microbial saccharification of all the chemically pretreated substrates showed an improved conversion of cellulose to glucose because of lignin removal during pretreatments. The alkali pretreated substrate caused higher microbial saccharification, which could be because of comparatively lower lignin content in the pretreated substrate. Neutralisation method for detoxification was observed to be better than the other employed detoxification strategy. The enzymatic saccharification when carried out with 20 UFPase per gram dry substrate resulted in optimum saccharification after 36 hour of incubation. The delignified substrate was subjected to microbial saccharification for the generation of fermentable sugars. To achieve the maximum saccharification of cellulosics OFAT approach was adapted. Various parameters including pH, temperature and agitation were studied. |
| Among various temperature range tested, the maximum microbial saccharification of delignified cellulosic biomass was achieved at 30°C. The results revealed that pH 5.5 was the optimum pH for the saccharification of delignified cellulosic substrates. The study revealed that all the nonionic surfactants showed significant increase (~40 %) in the extent of saccharification and among them Tween 80 caused maximum enhancement. Among various concentrations used, saccharification with 5ml was observed to release the optimum amount of sugars (184.96 mg/g). The fermentation process would be economically viable only if both hexose and pentose sugars present in the hydrolysates are converted to ethanol. S. cerevisiae has already been accepted as the most promising yeast for pentose fermentation, as it produced considerably more ethanol from pentose and hexose sugar mixture, showed higher volumetric rate and yield of ethanol production[30], [31] |
| Production of ethanol by S. cerevisiae was found to be dependent on pH, with maximum ethanol production at pH 5.5 and lowering or elevating the pH of medium adversely affect the ethanol production. Incubation temperature also plays a very prominent role for the production of ethanol using yeasts and in the present study the yeast produce maximum ethanol at 30°C. The results revealed that a combination of 1.2% soybean meal enhanced the ethanol productivity significantly by reducing the fermentation time. |
STATISTICAL ANALYSIS |
| Statistical tools were applied at various stages in this study for validation of the results obtained. The correlation coefficient of various factors studied at the saccharification stage were taken. Following are the corresponding results. |
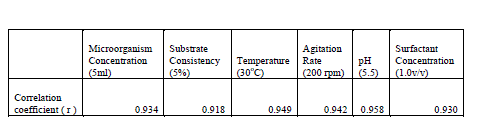 |
CONCLUSION |
| In the present study, bioethanol production process from lignocellulosic biomass i.e, Rice husk was developed. In order to fraction the lignin and hemicellulose from the lignocellulosic substrate, alkali and sodium chlorite pretreatment was adopted, which offers the possibility of producing cellulosic material that eventually will be highly amenable for microbial enzymatic hydrolysis. A detoxification strategy was also developed to eliminate the fermentation inhibitors from the hydrolysates. The delignified substrate was then hydrolyzed by commercial enzymes and microorganism. The results obtained with direct treatment with enzymes had faster and higher results than with microorganisms, but was not cost effective. Therefore the saccharification was taken forward with Trichoderma reesei . |
ACKNOWLEDGEMENT |
| The authors thank Prof. B.S. Satyanarayana, Principal R.V. College of Engineering for his constant encouragement and support throughout the research work. |
References |
|