ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
*V.R. Murthy and **A. Raghavendra Rao.
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
A glance at the data on densities of Alkyl Halides (C n H2n-1 (x)) suggests a gradual and hormonical increase in densities from Methyl Halides to Butyl and higher halides. This has resulted in an algebraic formulation of evaluation of densities of higher homologues from a given data on densities of Methyl Halides. Similarly the investigations in other Methyl Alcohols, Alkanes and Aryl halides have been discussed with relevant modifications of the Algebraic expressions. An extension to this method of calculation of densities is being attempted for Oligomers and Polymers.
Keywords |
| Density, Methyl Halides, Butyl halides, Methyl Alcohols, Alkanes. |
INTRODUCTION |
| The developments of atomic model from hydrogen to the higher elements in periodic table show a gradual induction of a proton and neutron into the nucleus and an electron in the outer valence shells. Any physical properties of these elements can be attributed to the number of electrons available in the outer valance shell. The formation of molecule from the combination of these atoms shows sharing of electrons in the outer valence shells of molecule. Hence the physical properties like density, refractivity...etc. can be interpreted in terms of response of these electrons to the electromagnetic radiation, electric field…etc. This basis of atomic and molecular formation result in gradual variation in physical properties like density, refractive index in lower homologous like methyl chloride to higher homologues (order) in terms of electronic response . An attempt is made in presenting the lower homologues as the fundamental unit in evaluating the properties of higher series. This additive properties in simple homologue is taken as a starting point to discuss the gradual variation of physical properties of Oligomers and polymers in term of monomer. |
FORMULATION OF RELATION AND EXPLANATION |
| A study of physical properties like density in methyl chloride and higher homologues indicated the variation in a gradual manner depending on electro negativity differences of the substituent element X, in the place of halide. A dependence of the density on the proportion of the outer valance electron in relation to total number of electron as reflected by un saturation factor is also noted .For example an addition of one electron resulting in a change from 2S to 2p brings forth variation in the electronic affinity and its response to physical properties. Incorporating all these changes an algebraic relationship is simulated to get at the densities of higher alkyl halides from that of methyl chloride. |
| A. Formula for alkyl halides: Taking alkyl chloride as Standard |
| Taking alkyl chloride as starting point the density of alkyl halides is expressed in simple formula. |
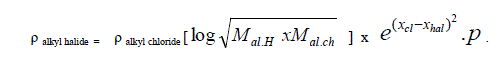 |
| Where |
| X hal and X cl are electro negativities of halogen and chlorine, M is molecular weight and p stands for unsaturation factor and it is decreasing from 1 to 0.6 with increase in homologous substitution. p values change gradually from 1 to 0.6, Accordingly p=1 for chloride , p=0.85 for bromide |
| B. Formula for alkyl alcohols taking alkyl chloride as standard. |
| Taking methyl alcohol as starting point the density of Alkyl Alcohols is expressed in simple formula. |
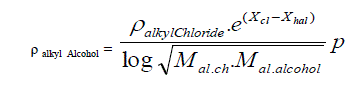 |
| Where |
| X hal and X cl are electro negativities and M stands of molecular weight, p is the un saturation factor and it is increasing from 1 to 1.35 with increasing the homologous substituent p=1, 1.05, 1.25, and 1.35. according as it refers to methyl alcohol, ethyl alcohol, propyle alcohol and butyl alcohols |
| C. Formula for Alkanes taking alkyl chlorides as standard. |
| Taking alkyl chloride as starting point the density of Alkanes is expressed in simple formula. |
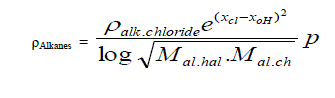 |
| Where |
| X cl and X 0H are electro negativities and M is molecular weight and p stands for un- saturation factor and its values change |
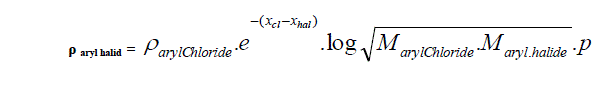 |
| D. Formula for Aryl halides taking Aryl chloride as standard. |
| Taking aryl chloride as starting point the density of aryl halides is expressed in simple formula. |
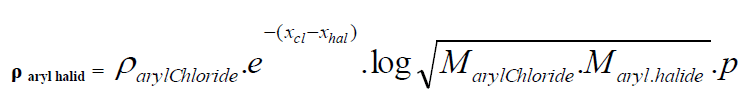 |
| Where |
| Xhal and Xcl are electro negativities and M is the molecular weight p stands for un-saturation factor and its value is 0.8 for benzyl series. |
RESULTS AND DISCUSSION |
| The algebraic relations formulated for the halides of Alkanes, alcohols of Alkanes, alkenes and aryl systems have been found to give satisfactory results. However, an increase in densities with molecular weight in these systems is confined only to lower homologues alone. In a given series of Alkyl Homologues when the molecular weight of methyl group is comparable to that of substituent group, an increase in density of the whole molecule is observed. On the other hand, when the molecular weight of methyl group is small and becoming smaller and smaller compared to the molecular weight of the substituent, a decrease in density is observed. The density variations in various homologues are also given in graph. For a given series of Bromides the density goes on decreasing with an increasing in the molecular weight of the substituent group. A similar future is observed in Chloride and Iodide, The density is decreasing in common from chloride to iodide. Similarly for the given methyl series the density goes on increasing with the atomic/molecular weight of substituent. For methane density goes on increasing from methane to methyl Iodide with the increasing in substituent with Hydrogen to Iodine. A similar future is observed in ethyl propyl and Butyl Series. |
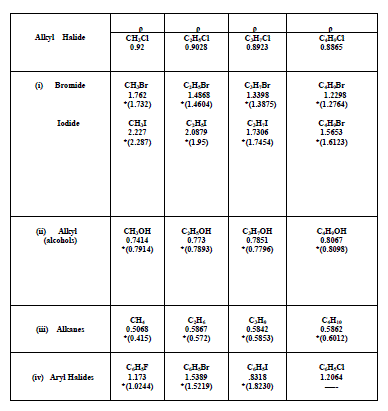 |
| *.The reported values of the densities are given in parenthesis. They are taken from reference |
Graph |
| Molecular Weight Verses Density of Alkyl Bromide, Alkyl Iodide, Alkyl and Aryl halides. |
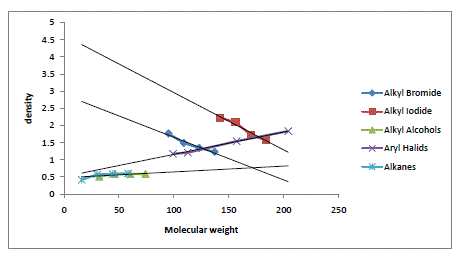 |
References |
|