e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
University College of Pharmaceutical Sciences, Kakatiya University, Warangal -506009, Andhra Pradesh, India.
Received: 23/03/2013 Accepted: 14/04/2013
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
A biphasic gastroretentive floating drug delivery system with multiple-unit pellets prepared by extrusion–spheronization process based on gas formation technique was developed for Alfuzosin Hydrochloride to maintain constant plasma level of a drug concentration within the therapeutic window. The system consists of a multiple - unit pellets to be filled into a hard gelatine capsule. The effect of the preparative parameters, e.g., Amount of PR coating on core units, amount of the effervescent agent layered onto the PR coated pellets, and type and coating level of the gas-entrapped polymeric membrane, on the floating ability and drug release properties of the multiple-unit FDDS were evaluated. The system using Eudragit RL30D, Eudragit RS30D and combination of them as polymeric layer could float within acceptable time. The drug release was linear with the square root of time. The rapid floating and the controlled release properties were achieved in this present study. The stability studies of samples showed no significant change in dissolution profiles. In vivo gastric residence time was examined by radiograms, and it was observed that the units remained in the stomach for about 6 h in fed state and stable for 6 months at 40°C/75% RH.
Floating drug delivery system; Pellets; Effervescent agent; Polymeric membrane; Controlled release.
Most of the floating systems previously reported are single unit systems such as tablets and capsules. A drawback of these systems is the high variability of the GI transit time due to their all-or-nothing emptying processes [1-4]. On the other hand, the multiple-unit dosage forms may be an attractive alternative since they have been shown to reduce the inter-and intra-subject variabilities in drug absorption as well as to lower the possibility of dose dumping [5-7]. Various multiple-unit floating systems have been developed in different forms and principles such as air compartment multiple-unit system [8], hollow microspheres (microballoons) prepared by the emulsion solvent diffusion method [9-12], microparticles based on low-density foam powder [3,13], beads prepared by emulsion–gelation method [14,15]. Recently, Choi et al. [16] prepared floating alginate beads using gas forming agents (calcium carbonate and sodium bicarbonate). Lingam et al developed a gastro retentive floating drug delivery system with multiple-unit mini tablets based on gas formation technique. In this core mini tablets were coated with three successive layers of an inner seal coat, effervescent layer (sodium bicarbonate) and an outer gas-entrapping polymeric membrane of an polymethacrylates (Eudragit RL30D, RS30D, and combinations of them) [17-19]. Extrusion spheronization is the most commonly used technique for pelletization [20]. Extrusion spheronization is a process of wet-extrusion, followed by spheronization, used to produce a wide variety of engineered, controlled release drugs. The excipients for pelletization by extrusion/spheronization are very limited because of the characteristics desired from wet masses. The main desired property for the extrusion process is to form a cohesive plastic mass on wetting [21,22]. In the most bead formulations, microcrystalline cellulose (MCC), commercially available as Avicel® and Emcocel® products, is regarded as a concial diluent and spheronizing aid for successful extrusion and spheronization [23]. Alfuzosin hydrochloride is a freely soluble weakly basic drug and used as an alpha-adrenergic receptor blocker approved by FDA for the treatment of symptomatic prostatic hyperplasia (BPH). Alfuzosin hydrochloride extended-release tablet is currently marketed under the brand name Uroxatral®, 10mg gastro-retentive controlled release dosage form.
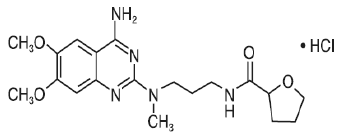
Alfuzosin shows linear kinetics when administrated at doses up to 30 mg daily. The absolute bioavailability of Alfuzosin is about 49% under fed condition, while the corresponding value under fasting condition is around 25% [24]. This shows that food has a significant impact on the oral absorption of Alfuzosin, potentially through the prolongation of gastric residence time. Moreover, Alfuzosin is preferentially absorbed in the proximal part of the gastrointestinal tract and, in particular, jejunum appear to be the main region for absorption [25]. As a result, prolongation of gastric residence time with a floatable controlled release gastro-retentive system allows continuous delivery from stomach to the intestine.
In this study pellets were filled into hard gelatine capsules, comprising of non-floating loading dose component and floating prolonged release component based on gas formation technique was developed. The drug containing core pellets was prepared by extrusion and spheronization method followed by coating of the prolonged-release units with prolonged release/seal coating, effervescent layer, and gas-entrapping polymeric membrane (Eudragit RS30D and RL30D). The effect of the preparative parameters like amount of the prolonged release coating onto the core pellets, effervescent agent layered onto the prolonged release coated units, type and coating level of the gas-entrapping polymeric membrane on the floating ability and drug release properties of the multiple-unit FDDS were evaluated.
Alfuzosin was received from Orchid laboratories and microcrystalline cellulose (MCC) (Avicel PH102) was a gift sample from AET laboratories Pvt. Ltd, Hyderabad, India. Methocel E 5 and ethyl cellulose 7 cPs were received as gift samples from Colorcon Asia Pvt Ltd (Goa, India). Sodium bicarbonate (Merk, India) was used as an effervescent agent with HPMC (Methocel E15LV), plasticized with polyethylene glycol 6000 (PEG 6000 Merck, India) as a binder. The gas entrapping polymeric membrane used was polymethacrylates (Eudragit RL and RS, Rohm Pharma, Germany) plasticized with triethyl citrate (Himedia), a water soluble plasticizer. All other reagents were of analytical grade.
Drug-containing core pellets were prepared by extrusion– spheronization process. Core pellets were prepared by sifting of all ingredients (Dry mix) mentioned in Table-1 through ASTM 40 mesh and mixed for 5 minutes in Rapid Mixer Granulator (HSMG10 Kevin Process Tech Pvt Ltd. India). The dry mix was granulated with purified water as binder to get suitable wet mass. The wet mass was extruded using 1.00 mm orifice in Extruder (Extruder 20, Caleva, ENGLAND) and the extrudates were immediately spheronized (Spheronizer 250, Caleva, ENGLAND) at 1000 rpm for 5 minutes. The obtained wet round pellets were dried in Rapid Drier (Restech drier TG 200, Restech, Germany) at 60°C±5°C to get loss on drying (LOD) at 105°C less than 2% w/w. The dried pellets were sifted and the fraction of ASTM 18 mesh passed and ASTM 35 mesh retains were taken for further coating. The composition was shown in table 1.
The core pellets were coated with three successive layers; a PR/protective layer, effervescent substance (sodium bicarbonate) as an inner effervescent layer and aqueous colloidal polymethacrylate dispersion (Eudragit® RL 30D, RS 30D or NE 30D) as an outer gas-entrapping polymeric membrane.
The calculated amount of Hypromellose (Methocel® E5 – LV Premium) and Ethyl cellulose 7 cP were dissolved in required quantity IPA. To this calculated amount of purified water is added and mixed for 60 minutes. The dispersion was made to contain solids of 8 % w/w. The above coating mixture is coated on to core pellets in Fluid bed coater (GPCG 1.1, Wurster Insert, Pamm Glatt India Pvt., India). The coated pellets were dried in an oven at 60°C for 12 hours.
and then layered onto the Prolonged release coated pellets. On a dry solid basis, the ratios of sodium bicarbonate to HPMC were 2:8, 5:5 and 8:2 w/w. The coating level of effervescent layer was 10% weight gain and the solid content of coating solution was kept constant at 12% (w/w).
The coating solution was sprayed onto the core pellets. The conditions for layering were shown as follows: bead charge, 300 g; preheating temperature, 50°C; preheating time, 20 min; inlet temperature, 50°C; outlet temperature, 40–42°C; atomizing air pressure, 25 lb/in2; spray rate, 8–10 ml/min. The sodium bi carbonate (NaHCO3)-layered pellets were dried in the coating chamber for 30 min at 50°C to evaporate the residual moisture. The prepared pellets were then removed from the coating chamber and stored in a closed container for further experiments. The sodium bi carbonate (NaHCO3)-layered pellets were subsequently coated with an aqueous colloidal polymethacrylate dispersion (Eudragit® RL 30D, RS 30D, or NE 30D) to achieve a weight gain of 5 and 10% (w/w) to obtain the complete multiple-unit FDDS.
A plasticizer (Di butyl phthalate or Tri ethyl citrate; 20%, w/w based on polymer solids) was added into the colloidal polymer dispersions (Eudragit® RL 30D, RS 30D) and the dispersions were gently agitated for at least 30 min prior to an appropriate dilution with purified water and subsequent coating. Eudragit® NE 30D can form film without the need of a plasticizer and thus diluted with water without the incorporation of a plasticizer. The solid content of the coating dispersions was 15% (w/w). The coating conditions were as follows: bead charge, 300 g; preheating temperature, 45°C; preheating time, 20 min; inlet temperature, 45°C; outlet temperature, 40–42°C; atomizing air pressure, 25 lb/in2; spray rate, 3–5 ml/min. The pellets were further dried in the coating chamber for 60 min after the coating was finished in order to evaporate the residual moisture in the polymeric coatings prior to storage. The dried pellets were filled in to capsules and analysed.
The biphasic GRDDS consists of non-floating loading dose component (15% core units) (NFLDC) and floating prolonged-release component (85% polymeric coated units) (FPRC) filled into a size “2” hard gelatine capsule.
The particle size distribution of core pellets was evaluated by sieve analysis. Two hundred grams of the core pellets were sieved through a set of sieves on a vibratory Sieve shaker (Retsch® Model AS 200 digit, Retsch, Germany) for 20 min, and the weight distribution was determined.
The friability of the core pellets was determined as the percentage of weight loss after 200 revolutions of 10 g of the core pellets in a friabilator (Electrolab; Model EF-2 Friabilator (USP).
For analysis of drug and drug–excipient interaction study, a differential scanning calorimeter (DSC 823e, Mettler Toledo) was used. Individual samples (drug and excipients) as well as mixtures of drug and selected excipients were taken in the pierced DSC aluminum pan and scanned in the temperature range of 80–330 °C (at the heating rate of 5 °C min−1) under an atmosphere of dry nitrogen.
The core, effervescent layered and final coated pellets were mounted onto the stage after coating with gold under vacuum. The surface morphology for checking the uniform coating of the units was observed under SEM
The floating abilities of the effervescent-layered pellets and the coated effervescent-layered pellets (complete multiple-unit FDDS) were determined using USP Dissolution apparatus type II (50 rpm, 37±0.2 ◦C, 900 ml, 0.1N HCl). Twenty pellets were placed in the medium; the time to float and duration of floating (floating time) were measured by visual observation. The percentage of floating pellets was calculated by the following equation:
Percentage floating of pellets =NMT/IN X 100
NMT = Number of floating pellets at the measure time
IN = Initial number of the pellets
Contents of 20 capsules were weighed and finely powdered. Transferred an accurately weighed portion of the powder, equivalent to about 40 mg of Alfuzosin HCl to a 500-ml volumetric flask and added 50 ml of 0.1 N hydrochloric acid, and sonicated to dissolve it. Shaken by mechanical means for 10 minutes, diluted with water to volume, mixed, and passed through a filter having a 0.5μm or finer porosity. Drug content was determined by using UV Visible Spectrophotometer at 244nm.
In-vitro drug release studies were carried out for extended release Alfuzosin HCl formulations using 0.01N HCl as dissolution medium using USP Apparatus-I (basket) at 100 rpm (Electrolab, 2000) and the temperature was maintained at 37±0.5°C. The dissolution was continued for 24 hours while samples of 5 ml were withdrawn at regular interval and replaced with equal volume of fresh dissolution medium to maintain the volume constant. The samples were filtered, diluted and analysed for drug content. The amount of drug released was determined by UV spectrophotometer at 244nm. Drug release at specified time points was calculated.
In the model independent method, the dissolution data are fitted into relevant selected mathematical models, characterized by suitable mathematical functions. The dissolution profiles are then evaluated in terms of the derived model parameters. Mathematical models used in dissolution data analysis include the zero order rate equation, which describes the systems where the release rate is independent of concentration of the dissolved species [26]. The first order equation describes the release from systems where dissolution rates dependent on the concentration of the dissolving species [27]. The Higuchi square root equation describes the release from systems where the solid drug is dispersed in an insoluble matrix and the rate of drug release is related to the rate of drug diffusion [28-29]. To gain some insight into the drug release mechanism, where n=0.5 indicating diffusional controlled drug release, and n=1.0 indicating swelling controlled drug release and values n between 0.5 and 1.0 can be regarded as indicating the superposition of both phenomena (anomalous transport) [30]. The applicability of all of these equations was tested in this work.
The model independent method compares dissolution data directly and does not rely on the choice of mathematical model which may be unrealistic at times. In this method, graphical representation of the dissolution profiles is performed as a preliminary step to illustrate non-quantitative differences and their evolution along the profile. The dissolution data are subjected to further analysis, using time-point or pair wise approaches to determine similarity (F2) between dissolution curves. The dissolution data was subjected for determining F2 values by using the formula:
F2 = 50 x log{1+1/n)E τ=1 n(Rt – Tt)2}-0.5 x 100
The In-vivo tests were performed on six healthy male volunteers whose ages were between 25 and 32 years and weighed between 60 and 71 kg (approval for the study was taken from University Ethical Committee, UCPSc, Kakatiya University, Warangal, AP, India); 20% of BaSO4 was added to the part of the final formulation (the amount of BaSO4 that allows visibility by X-ray, but does not preclude the floating of tablets, was experimentally determined). Labelled Floating MUDF (Placebo) was given to subjects with 250 ml of water after a light, 308 kcal breakfast. Following ingestion, gastric radiography was undertaken at 0.5, 1, 3, 4 and 6 h, and the duration of the pellets stayed in the stomach was observed.
To assess the drug and formulation stability, stability studies were performed according to ICH and WHO guidelines (19, 23). Optimized formulation was kept in the humidity chamber (Lab Top, India) maintained at 40 °C and 75% Relative Humidity for 6 months. At the end of studies, samples were analyzed for physicochemical parameters.
The design of multiple-unit FDDS was shown in Figure I.
The system consists of drug-containing core pellets coated with PR coating layer, effervescent layer and gas-entrapping polymeric membrane, respectively. Since sodium bicarbonate itself could not adhere onto the core pellets, HPMC was used as a binder in the inner effervescent layer.
An ideal coating material for a floating system should be highly water permeable in order to initiate the effervescent reaction and the floating process rapidly. However, the wet or hydrated coatings should also be impermeable to the generated CO2 so as to promote and maintain floatation [31]. Regarding their mechanical properties, the polymeric coatings should be sufficiently flexible in wet state to be able to withstand the pressure of the generated gas and to avoid rupturing. Krogel and Bodmeier (1999) [31] reported that the cellulosic polymers were not suitable candidates for FDDS. Cellulose acetate was too rigid and did not expand sufficiently when in contact with dissolution media, while ethyl cellulose was not flexible and ruptured easily upon CO2 formation. Gas bubbles were released rapidly after the burst of coating. Due to these reasons, the higher flexibility polymer, an aqueous colloidal polymethacrylate dispersion (Eudragit® RL30D, RS30D, or NE 30D), was chosen and investigated as a gas-entrapping polymeric membrane in this study. Upon contact with the gastric fluid, the fluid permeated into the effervescent layer through the outer polymeric membrane. Carbon dioxide was liberated via neutralization reaction and was entrapping in the polymeric membrane. After that, the swollen pellets (like balloons) with a density less than 1.0 g/ml floated and maintained the buoyancy; therefore, the drug was released from the system for a long time. To develop the multi-unit FDDS based on gas formation technique, several studies were necessary to identify the formulation variables providing the desired system properties, rapid expansion and formation of low-density system within minutes after contact with gastric fluids and maintaining the buoyancy in stomach with sustained release. The effect of the preparative parameters such as amount of PR coating onto the core pellets, amount of the effervescent agent layered onto the PR coated pellets, and type and coating level of the polymeric membrane, on the floating ability and drug release of the multiple-unit FDDS were evaluated.
The formulations were evaluated for pharmacopoeial quality control tests and all the physical parameters evaluated were within the acceptable limits. The friability of the formulation was 0.18±0.05%. This indicated that the core pellets were quite hard and able to withstand the mechanical stresses of the subsequent coating process. The drug-containing core pellets obtained by the extrusion–spheronization showed narrow size distribution and the dominant size fraction was 0.80–1.00 mm. The appearance of the core pellets were done by visual/physical observation. The core pellets were spherical agglomerates with a slightly rough surface. The surface of the effervescent-layered pellet was slightly smoother and the smoothest was the surface of effervescent-layered pellet coated with polymeric membrane (Eudragit® RL 30D).
The DSC thermogram of the pure drug, excipients, physical mixture of drug and polymer which was kept at 40°C±2°C/75%RH±5%RH revealed that an endothermic peak of melting of drug appears at about 225-231°C indicating that there was no incompatibility between drug and excipients (Figure II).
Figure III a shows the external morphology of the core units under SEM. The core units were with a slightly rough surface. The surface of the effervescent layered units was slightly smoother (Figure. IIIb) and the smoothest was the surface of effervescent-layered units coated with polymeric membrane (Eudragit RL30D/RS30D) (Figure. IIIc).
The floating ability of the effervescent-layered units and the effervescent-layered units coated with polymeric membrane (complete multiple-unit FDDS) were investigated with respect to amount of the effervescent agent coated, and type and level of the polymeric coating. The system should float within a few minutes after contact with gastric fluid to prevent the dosage form from transiting into the small intestine together with food. The percent coating level of effervescent layer was evaluated and found to be 10–12% for floating. The effervescent layered units floated within 5–10 seconds after placing in 0.1 N HCl . The floating time of the effervescent-layered units was quite short (less than 3 h) because HPMC dissolved and there was no polymeric membrane which could entrap the generated CO2 gas. Therefore, the complete multiple-unit FDDS (effervescent layered units coated with polymeric membrane) were prepared and evaluated for floating ability.
Eudragit RL30D, RS30D and in combination were used as polymeric membrane. The multiple-unit FDDS using Eudragit RL30D and Eudragit RS: RL30D as polymeric membranes floated completely within 3 min. The time to float of the systems decreased with increasing amount of effervescent agent and increased with increasing level of polymeric membrane coating (Figure IV). The higher amount of effervescent agent caused faster and higher CO2 generation [31]. With increasing level of Eudragit RL30D, the floating was delayed due to slow water penetration through the thicker coating. The duration of floating was longer than 8 h. It was found that Eudragit RL30D and RS: RL combinations polymeric membrane was impermeable to the generated CO2 and could maintain the floatation. The multiple-unit FDDS systems coated with Eudragit RS30D as polymeric membranes did not float within 20 min even when used with high effervescent coating level (15% w/w weight gain). Eudragit RS30D might not be permeable enough for dissolution medium to induce the effervescent reaction and generate sufficient amount of CO2 to make the units float. Eudragit RL30D is a highly water permeable polymer due to its hydrophilic quaternary ammonium groups in the structure (Figure V) [32,33]. It has twice as many quaternary ammonium groups and is more hydrophilic than Eudragit RS. A faster and higher CO2 generation caused by increasing the level of effervescence resulted in higher swelling of polymeric membrane and subsequent floating. It is therefore hydrated faster and resulted in a shorter time to float. Based on these results, Eudragit RL30D and combination of RS: RL30D were the polymers of choice as gas-entrapping membrane in this multiple-unit FDDS.
The core pellets dissolution profile in 0.1N HCl showed immediate release (within 30 minutes getting more than 90% drug release) than these core pellets coated with Ethyl cellulose and HPMC (PR Coating) for controlling drug release. Increasing coating level (5-20%) of PR coating decreased the drug release (Fig No: VI). The optimized PR coating level is 15% weight gain. The optimized EC: HPMC ratio is 85:15 for controlling drug release. In the ratio of 65:35 fast release observed and in the ratio of 90:10 incomplete releases observed. (Fig No: VII)
Increasing coating level of gas-entrapping polymeric membrane decreased the drug release. Combination of Eudragit RL and Eudragit RS with 8 % coating level with different ratios shows less release than individual Eudragit RL 8% coating level (Fig No: VIII).
The correlation coefficient (r2) was used as indicator of the best fitting, for the models considered. The results (Table 2) reveal that all formulations of FDDS were best fitted in the zero order and Higuchi model.
The mechanism of drug release from these pellets was found to be diffusion controlled as seen from r2 values of Higuchi model. The n values for these systems from krossmeyer peppas equation was (0.8263) between 0.45–0.89, indicating both phenomena (transport corresponding to coupled drug diffusion in the hydrated matrix and polymer relaxation) commonly called anomalous non-Fickian transport.
The dissolution data of optimized formulation in 0.1 N HCl plotted in accordance with the zero-order equation is percent dissolved as a function of time (Figure.VIII). It is evident from the figure that the plots are linear, suggesting that the release process is zero-order in nature.
The drug release was sustained and linear with the square root of time. Both the rapid floating and the sustained release properties were achieved in the multiple-unit floating drug delivery system developed in the present study. Only the system using Eudragit® RL 30D as a gas-entrapping polymeric membrane could float.
The in vivo gastric residence time was examined by radiograms and it was observed that the units remained in the stomach for about 6 h in fed state (Figure.IX).
The analysis of the dissolution parameter data 0 day (Initial) after storage at 40°C±2°C/75%RH±5% for 6 months showed, no significant change indicating that the two dissolution profiles were considered to be similar (f2 value was initial-83 and ACC 6M-87, more than 50, Figure.X).
Comparative Release rate of Alfuzosin HCl floating pellets 10 mg in 0.1 N HCl showed, that the final formula at 0 day (Initial) and Accelerated stability condition 40°C±2°C/75%RH±5% (ACC 6M) release rate were similar to marketed product.
A biphasic gastroretentive floating drug delivery system with multiple-unit pellets prepared by extrusion–spheronization process based on gas formation technique was developed for Alfuzosin Hydrochloride to maintain constant plasma level of a drug concentration within the therapeutic window. The system consists of a multiple - unit pellets to be filled into a hard gelatine capsule. In this the pellets are divided into two parts one is non floating loading dose component as uncoated core units and second is prolonged-release component as coated units. The latter were coated with three successive layers, one of which is prolonged release/ seal coat, an effervescent layer, and an outer polymeric layer of polymethacrylates. The effect of the preparative parameters, e.g., Amount of PR coating on core units, amount of the effervescent agent layered onto the PR coated pellets, and type and coating level of the gas-entrapped polymeric membrane, on the floating ability and drug release properties of the multiple-unit FDDS were evaluated. The system using Eudragit RL30D, Eudragit RS30D and combination of them as polymeric layer could float within acceptable time. The drug release was linear with the square root of time. The rapid floating and the controlled release properties were achieved in this present study. The stability studies of samples showed no significant change in dissolution profiles. In vivo gastric residence time was examined by radiograms, and it was observed that the units remained in the stomach for about 6 h in fed state and stable for 6 months at 40°C/75% RH.
The multiple-unit FDDS based on gas formation technique was developed. The system consists of drug-containing cores coated with Prolonged Release/Protective coating, effervescent layer and polymeric membrane. The floating ability and drug release of the system were dependent on the amount of the prolonged release coating onto the core pellets, effervescent agent layered onto the prolonged release pellets, and type and coating level of the polymeric membrane. Only the system using Eudragit® RL 30D as a polymeric membrane could float as Eudragit® RL 30D had high water- and low carbon dioxide (CO2)-permeabilities with high flexibility. The system could float completely within 3 min and maintain the buoyancy over a period of 24 h. The multiple-unit FDDS with rapid floating and sustained drug release was obtained and could be a promising gastroretentive DDS. Based on the comparative release rate of Alfuzosin HCl floating pellets 10 mg in 0.1 N HCl (initial and ACC 6M) with marketed product the final formula was selected for further In-vivo study. In- Vivo study in humans showed that the multiple unit FDDS remained in stomach for 6 hrs in fed condition.
The authors would like to thank Orchid chemicals and Pharmaceuticals Ltd (Chennai, India) for providing the gift sample of Alfuzosin hydrochloride, and one of the author (K. Vinay Kumar) is thankful to the AET Laboratories Pvt. Ltd (Hyderabad, India) providing facilities for doing pelletization (extrusion and spheronization) and coating throughout my research work.