Keywords
|
| Non-Proliferative Diabetic Retinopathy (NPDR), Fundus image, Retinal Hemorrhage, Splat feature, Gray Level Co-Occurrences Matrix (GLCM). |
INTRODUCTION
|
| Diabetic Retinopathy (DR) is one of the eye diseases that occur due to hypertension, blockage of retinal vein, damage of blood vessels. Various kinds of abnormalities are present to find these diseases that are micro aneurysms, hard & soft exudates, Retinal hemorrhage, macular edema, lesion and cotton wool spots. Retinal hemorrhage (i.e.), bleeding occurs into the retensitive tissue in the black wall of the eye. It is caused by retinal vein occlusion, diabetes mellitus. DR has two types. First, Earlier stage is Non- Proliferative Diabetic Retinopathy (NPDR) in which symptoms will be mild or non-existent that occur due to small amount of bleeding and fluid leaking into retina. Retinal hemorrhage [1] is useful to find NPDR. Second, advanced (or) severe stage is Proliferative Diabetic Retinopathy (PDR) occurs due to new blood vessel starting to grow in the eye that are fragile and can bleed. It becomes Blindness. |
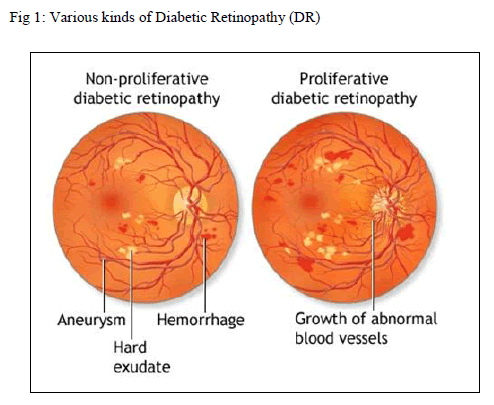 |
| Two kinds of DR are shown in Fig 1. So, earlier detection of NPDR is helpful to improve automated screening system. DR is sight threatening complication. There are several level of DR severity [2] that are mild, moderative, and severe. At first, the people suffering with DR may notice no changes in their vision. It could get worse over the years and threaten their good vision. Treatment for diabetic retinopathy depends on the stage of the disease and is directed at trying to slow or stop the progression of the disease. |
| Clinical reference standard [3] is used to detect various signs of DR. Diabetes affection does not necessarily involve vision impairment, about 2% of the patients affected by this disorder are blind and 10% undergo vision degradation after 15 years of diabetes as a consequence of DR complications. The estimated prevalence of diabetes for all age groups worldwide was 2.8% in 2000 and 4.4% in 2030, meaning that the total number of diabetes patients is forecasted to rise from 171 million in 2000 to 366 million in 2030. |
| Ophthalmoscopy is a reasonable screening method when performed by well-trained personnel on dilated fundi. , which may improve retinopathy screening in areas with a shortage of eye care specialists. Screening [4] for diabetic retinopathy (DR) is important because the majority of patients who develop DR have no symptoms until retinal hemorrhage is already present. So Early detection of DR through screening can prevent blindness and allow for maintenance of good vision. The rest of the paper is organized as follows: Section 2 summarize the overview of the related works. Section 3 depicts about the proposed framework. Section 4 describes the performance analysis of proposed work. Section 5 presents concluding remarks and outlines directions for future work. |
II. RELATED WORKS
|
| In the existing work, Based on the size of lesion [1] lesion based approach perform some morphological transformation to detect retinal hemorrhage with the help of pixel & image based approaches that focus the location of hemorrhage. The ensemble system [2] contains bagged and boosted decision tree, use supervised blood vessel segmentation method to perform orientation analysis to detect DR signs. To find exudates, automated method [3] use K- means clustering algorithm to extract the relevant features. With the help of accurate vessel segmentation Multi layered threshold technique [4] eliminates false edges and small vessel segment. To detect diabetes mellitus, DR Grading system [5] classifies disease severity by V fold cross validation method that performed on the retinal images from FINDERS DB. Rule based classifier define the retinal status [6] as normal and abnormal (mild, moderate, severe) on the STARE DB retinal images. Supervised Neural Network (NN) classify the pixel to detect the blood vessel [7] based on gray level and moment invariant features. To detect micro aneurysms and drusen detection algorithm [8] use the free response receiver that works with operating characteristic analysis. Quantitative technique also used to calculate the number of lesion. Abnormal retinal images [9] are detected by reducing the number of false negatives. The above approaches concentrate the following performance measure that are speed, sensitivity, accuracy, specificity, robustness, computation time and reduction of false positive and false negative. Still that is suffering with some limitations. Our proposed work focuses the abnormality called retinal hemorrhage in fundus retinal image set to extracting splat feature using support vector machine classifier for detection and improving the accuracy with reduction of FP and FN. |
III.SYSTEM DESIGN
|
| A Typical screening process [4] involves the acquisition of retinal images from the patient followed by manual examination of each individual image by medical experts in order to identify signs of DR. Compare to other abnormalities Retinal hemorrhage [1] is useful to detect NPDR. So, efficient classifier for automatic detection of DR are playing vital role in the field of medical image processing. This proposed technique endows with reliable method to detect the presence of retinal hemorrhage in digital fundus image to reduce the computation time. Acquired retinal images are pre-processed [5] and set of splat features are extracted. These features have been fed into the SVM classifier that are trained by supervised learning [7] with data from manually labeled features to detects the retinal hemorrhage and classifying the abnormality from retina. At the end, performance is evaluated by compare the classification accuracy with KNN. Modules of our proposed work are shown in Fig 2. |
| A. Image Acquisition |
| Retinal fundus images [1] are the interior surface of the eye specify opposite portion of lens include retina, optic disc, macula, fovea and posterior pole. These images should examine and verified by ophthalmoscopy [10]. Fundus images are collected from Messidor (Methods to evaluate segmentation and indexing technique in the field of diabetic retinopathy) data set. |
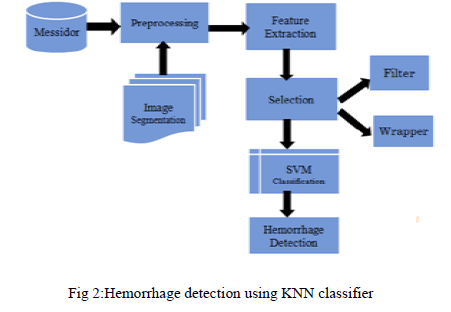 |
| It is one of the publically available dataset contains the images in the form of uncompressed tagged image file format with 1440 × 1960 pixel resolution that is about 4MB / image. These kinds of images are taken as input. |
| B.Preprocessing |
| Color fundus images that are collected from Messidor dataset are often show important lighting variations, poor contrast and noise. In order to reduce these imperfections and generate the images that are more suitable for extracting the pixel features, pre processing process [6] is mandatory. Pre-processing method use the small neighborhood of pixel in an input image to get the new brightness value in the output and it is performed based on the morphological filtering [10]. So, it is used to detect incorrect boundary. In this process, low frequency back ground noise is removed. Each individual particle is normalized. Then Reflection and masking portions are removed. Based on Tri-chromatic theory, color components such as R, G and B are separated. |
| C. Image Segmentation |
| Image segmentation [4] is the process of partitioning a digital image into multiple segments (i.e.) set of pixels. The goal of segmentation is to simplify and/or change the representation of an image into something that is more meaningful and easier to analyze. It is typically used to locate objects and boundaries. Then, assign a label to every pixel in an image such that pixels with the same label share certain visual characteristics. Part of pixel from same object (or) structure that share similar color, same intensity and spatial location. That kind of pixel group is called splat [1].Without overlapping those pixel particles are partitioned into meaningful splat segments that are performed after pre-processing. Based on scale-of-interest (SOI) and with the help of aggregated gradient magnitude, watershed segmentation is performed to identify GLCM features and preserve hemorrhage boundary. |
| D. Feature Extraction |
| When the input data is too large to be processed and it is suspected to be notoriously redundant. Then the input data will be transformed into a reduced representation set of features [11]. Transforming the input data into the set of features is called feature extraction. It is also called as dimensionality reduction.If the features extracted are carefully chosen it is expected that the features set will extract the relevant information from the input data in order to perform the desired task. |
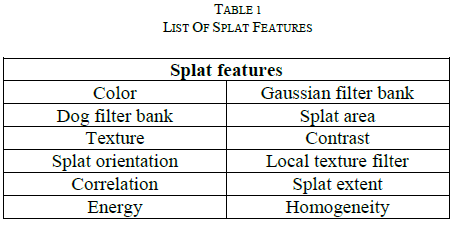 |
| Using this reduced representation instead of the full size input. In order to classify [9] the given input images different classes must be represented using relevant and significant features. Splat and GLCM features are extracted that are useful to detect retinal hemorrhage. 1) Splat Features: Splat features are extracted by individual splat distribution and aggregation of splat, based on pixel collection. Each individual extracted splat should describe its surrounding and interaction with neighbouring splats and texture information. Some of splat features are listed in Table 1. Some kind of splat features is color, splat area, texture, splat extent, splat orientation, Gaussian filter bank. Compare to the previous pixel based approaches [1], miss classification of features occur due to consider the single pixel.Here, focusing the splat (i.e.) Group of pixel and with the help of reference label, SVM classifier is trained to detect the hemorrhage by splat based feature vector |
| 2) Gray Level Co-Occurrence Matrix: These features mainly focus texture information. GLCM is a matrix where the number of rows and columns is equal to the number of grey levels [7]. The matrix element is relative frequency in which two pixel are separated by pixel distance one with intensity i and other with intensity j. Matrix element contains the second order statistical probability value for changes between i and j at a particular angle. A co-occurrence matrix C is defined over an n × m image I, parameterized by an offset (Δx, Δy), as |
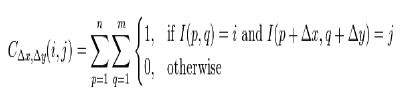 |
| Where i and j are the image intensity values of the image, p and q are the spatial positions in the image I and the offset (Δx, Δy) depends on the direction used and the distance at which the matrix is computed d. |
| E. Feature Selection |
| Feature set is selected such that discrimination between the classes is maximized while the feature within class [10] is minimized. After feature extraction relevant features are selected to reduce the feature space and make feature set to be small. This process is performed by two step selection process [12]. Those are Filter and Wrapper approaches. Filter approach is initial and necessary process. It is applicable to all high dimensional feature space and this method is very fast. The main goal of filter approach [8] is reduce the non-effective and irrelevant features to separate the hemorrhage and non-hemorrhage splats. After select the individual needed features, Wrapper approach select the different combination of feature subset based on the interaction and minimize the redundancy. |
| D. Classification |
| SVM is a binary linear classifier for classifying the training examples. It analyzes the data and recognizes patterns. So, it predicts the given input and makes the two possible classes and forms the output. Each training features are marked as belong to one of two categories. Finally it builds a model that assigns new features into one category (or) other. Classifier is trained by supervised learning methods [7]. Based on the vector value, Fundus images are finally classified in two categories, vector whose value 1 indicates hemorrhage affected retina and 0 indicates normal retina. It is helpful to detect the abnormal position. Assuming given some training data D, a set of n points of the form |
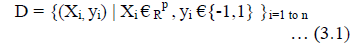 |
| Where yi is either 1 or -1, indicating the class to which the point xi belong each Xi is p dimensional real vector. W.X – b = 0 … (3.2) Maximum - margin - hyper plane (3.2) that divides the point yi = 1 from yi = –1 in the set of points X W. Xi – b ≥ 1 ... (3.3) If the training data are linearly separable, hyper planes are selected by separating the data using different classes that are represented by both (3.3) & (3.4) W. Xi – b ≤ 1 ... (3.4) The above two classes classify the hemorrhage affected retina and normal retina from retinal fundus images. |
IV. PERFORMANCE ANALYSIS
|
| A Classification model [9] is tested by applying it to test data with known target value and comparing the predicted values with known values. If the model performs well and meet the requirements then be applied to the new data to predict the future. Accuracy of a classifier acc refers to the percentage of correct predictions made by the model when compared with the actual classifications [1] in the test. Misclassification rate M is calculated by |
| M = 1- acc (M) … (4.1) |
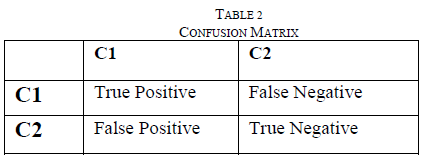
| Confusion matrix is used to calculate the performance measure. Confusion Matrix Table 2 shows the number of correct and incorrect prediction made by the model compared with the actual classifications in the data. Given m classes, CMij, an entry in a confusion matrix, indicates # of all the tuples in class i that are labeled by the classifier as class j. Alternative accuracy measures are calculated by |
| % Specificity = TN /TN + FP * 100 … (4.2) %Sensitivity = TP/ TP + FN * 100 … (4.3) Precision =TP/ (TP + FP) … (4.4) |
 |
| Finally classification accuracy is calculated by % Classifier accuracy = TP + TN / Number of total subjects |
V. RESULTS AND DISCUSSION
|
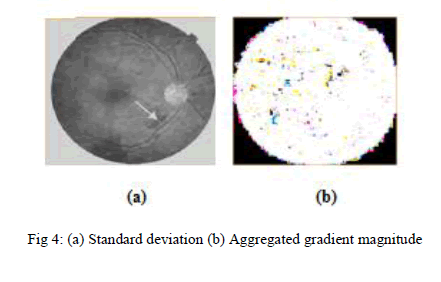
| Data sets of 30 retinal fundus images are tested. The results for each step explained in the proposed method are discussed in this section. The hemorrhage detection results for sample image of the MESSIDOR dataset are shown in Fig. 3.These images with 1440 × 1960 pixel resolution are taken as input for this process. To prepare an image for further processing after image acquisition, preprocessing is performed, that is shown in Fig 6. This enhancement is useful for find the standard deviation to extract the required features easily. To identify the blood and retinal in separate manner gradient magnitude of the contrast and enhanced dark bright images should aggregated because hemorrhage size and appearance are vary in different images. Gradient magnitude of the given image is shown in Fig 4.. |
 |
 |
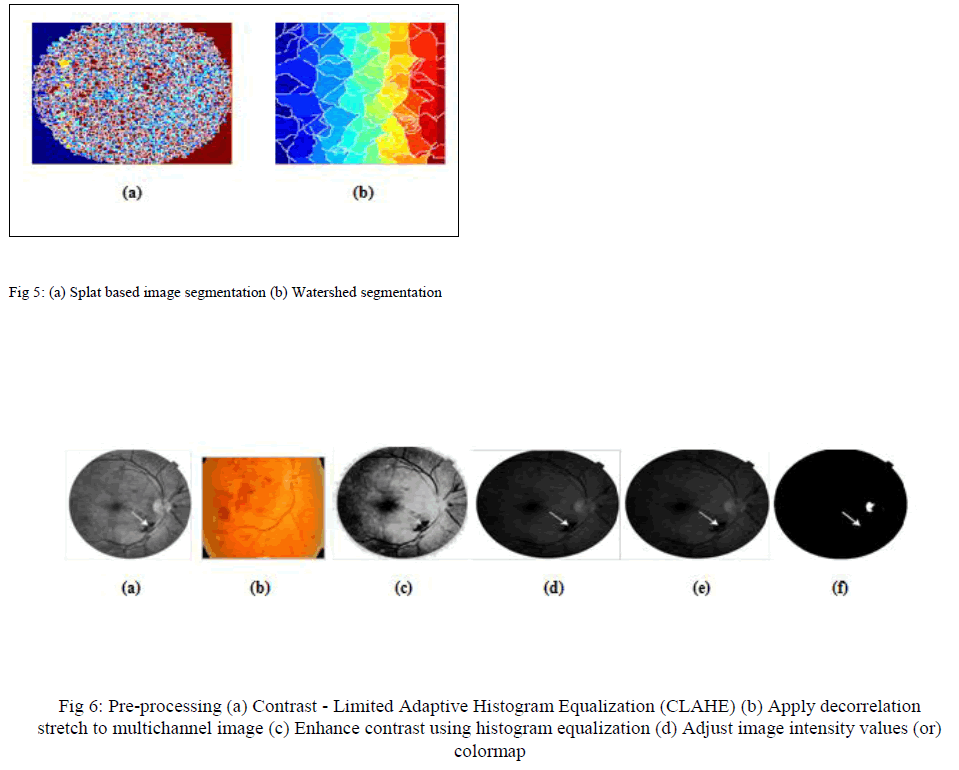 |
| Splat based image segmentation that creates a splat and watershed segmentation used to extract the GLCM feature are performed to partition the images into group of pixel segments. This should cover the entire image. Both segmentation processes is shown in Fig 5. |
| To extract the splat features Gaussian filter are enhanced and for GLCM features texture filter are enhanced from segmented images with the help of standard deviation. Both filters are shown in Fig 7 |
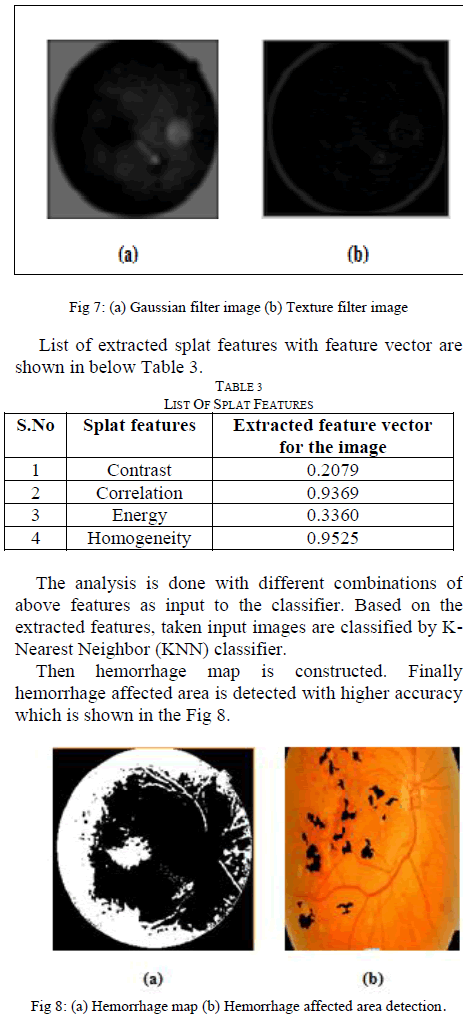 |
| Depends on the sensitivity and specificity measures classification accuracy is calculated that is compared to KNN classification both are shown in Fig 9. |
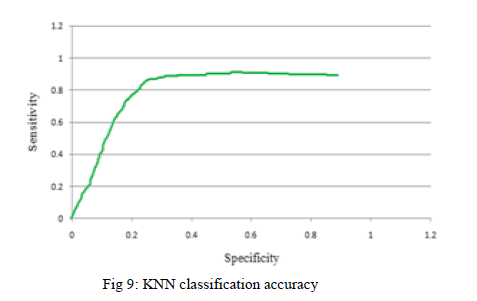 |
| The Area under the curve from the above Graphical representation specifies the KNN classifier accuracy to detect the retinal hemorrhage. |
IV. CO NCLUSIONS
|
| Eye vision plays vital role in our senses. Image mining is helpful to diagnosis of diseases. In this work, one of the eye disorder Diabetic Retinopathy is focused. The proposed technique elucidates to increase the performance of screening system [6], that diagnosis the DR at initial stage by detects the retinal hemorrhage. For this purpose, supervised SVM classifier is trained based on the number of relevant, extracted splat and GLCM features to classify the images into hemorrhage affected retina and normal retina from normal and abnormal retinal images to detect the retinal hemorrhage. This proposed work shows the KNN classification for hemorrhage detection. In future work this classification accuracy [1] is compared with SVM classifier. |
References
|
- Li Tang, Meindert Niemeyer, Joseph M. Reinhardt, Mona K.Garvin and Michael D. Abramoff., Splat Feature Classificationwith Application to Retinal Hemorrhage Detection in FundusImages, IEEE Transaction on Medical Imaging, Vol. 32, NO. 2,FEBUARY 2013
- Paolo Remagnino, Andreas Hoppe, BunyaritUyyanonvara,Alicja R., An Ensemble Classification-Based Approach Appliedto Retinal Blood Vessel Segmentation, IEEE Transaction onBiomedical Engineering, Vol. 59, NO. 9, SEP 2012.
- B.Ramasubramanian, G.Mahendran.,An Efficient IntegratedApproach for the detection of Exudates and DaibeticMaculopathy in color fundus images, Advanced Computing: AnInternational Journel (ACIJ), Vol.3, No.5, 2012.
- M.UsmanAkram, Shoab A, Khan.,Multilayered thresholdingbasedblood vessel segmentation for screening of DiabeticRetinopathy, Springer, DOI 10 .1007/0253-7,2012.”
- H. Ahmad Fadzil, Lila IznitaIzhar ,HermawanNugroho.,Analysis of Retinal Fundus Image for Grading of DiabeticRetinopathy Severity, Med BiolEngComput (2011) 49:693–700DOI 10.1007/s11517.
- Ahmed Wasif Reza and C. Eswaran,.,Detection support systemfor Automatic Screening of NPDR, Med Syst (2011) 35:17–24DOI 10.1007/s10916-009-9337-y.J.
- Diego Marin, Arturo Aquino, Manuel Emilio Gegundez-Arias,and Jose., A New Supervised Method for Blood VesselSegmentation in Retinal Images by Using Gray-Level andMoment Invariants-Based Features”, IEEE Transaction onMedical Imaging, Vol. 30, NO. 1, JAN 2011
- GwenoleQuellec, Stephen R. Russell, and MichaelD.Abramoff.,Optimal Filter Framework for Automated,Instantaneous Detection of Lesions in Retinal Images, IEEETransaction on Medical Imaging, Vol. 30, NO. 2, FEB 2011
- J.Anitha, C.KeziSelvaVijila, D.JudeHemanth and A.Ahsina D.,Self organizing Neural Network Based pathology Classificationin Retinal Images, IEEE Transaction,978-1-4244-5612-3/09,3009.
- François Mendels, ConorHeneghan,Jean-Philipp.,Identificationof the Optic Disk Boundary in Retinal Images using ActiveContours, World Health Organization (WHO) PublicationPBL/87.14 (Update 1987),Available Data on Blindness. WHO,Geneva, Switzerland, 1987
- Huiqi Li, Chutatape, O.GwenoleQuellec, Automated featureextraction in color retinal images by a model based approach,IEEE Transaction on Medical Imaging, Vol. 51, NO. 2, FEB2004
- M. Dash, H.Liu,.,Feature Selection for classification. IntelligentData Analysis 1 (1997) 131–156
|