e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
Chaudhari Kanchan S*
Department of Pharmaceutics, MET’S Institute of Pharmacy, Adgaon, Nashik, Maharashtra, India
Received date: 03/12/2021; Accepted date: 17/12/2021; Published date: 24/12/2021
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
Objective of present study was to formulate directly compressible fast disintegrating tablet for water purification and flocculation of suspended solids present in the water. Concentration of flocculent, effect of stirring rate is study for flocculation of water. Tablet was evaluated for weight variation, thickness, hardness, friability, in vitro disintegrating time, wetting time. A 32 full factorial design was applied to investigate the concentration of disintegrating and flocculent required. The water was evaluated for TDS, Turbidity, PH, conductance and content of aluminum. The tentative outcome from all the result show that the optimum concentration of super disintegrants and concentration of flocculent gives expected results. The formulation as dispersible tablet will help in avoiding confusion in deciding concentration of flocculent and avoids variation. From All the evaluated data it was concluded that tablet is used for water purification during trekking, climbing, adventure trip in unknown place.
Water is utilized for various purposes like drinking, Washing, Bathing, Recreation as well as numerous other industrial applications. According to World Health Organization WHO (1971), reports that wholesomeness of water means absence of suspended solids, inorganic solids and pathogens [1].
Water covers 71% of earth’s surface, (CIA-The World Facebook, 2008) on earth, 96.5% of the planet’s water is found in oceans, 1.7% is ground water, 1.7% in glaciers and ice caps of Antarctica and Greenland, a small fraction in other large water bodies and 0.003% in the vapor clouds. Water purification tablets are simple and portable. Properly stored, many types of tablets remain effective for months or years. Unlike purification techniques involving boiling, filtration or ultraviolet radiation, water purification tablets require no special equipment, power or fuel. Water purification tablets are frequently used as a backup for other techniques in case of equipment failure. Because water purification tablets are small and lightweight, they are often included in survival and emergency kits [2].
MO was popularly used as folk medicine for anemia, arthritis and rheumatism, asthma, constipation, diarrhea, stomach pain, Ulcer, intestinal spasms, headache, sore gums. Various preformulation studies shows MO gum has mucoadhesive, disintegrant, and binding property. Many study concluded that MO is an excellent natural source natural source for pharmaceutical excipient.
In present study Alum and MO is used as coagulant or flocculant for water purification [3]. Various types of water purification tablet are available in the market [4]. The active ingredient present in marketed water purification tablet includes iodine, chlorine etc. This ingredient having disinfectant action, but they unable to form flocs in the water [5]. So it cannot remove particulate water from the water. So, objective of the study was to developed tablet formulation for water purification [6].These tablet contain Alum and MO powder as an active ingredient which gives clear water and MO seed powder having antimicrobial activity.
The antimicrobial activity of the MO seed powder was studied with the help of Agar disk-diffusion method. Tablet formulation is prepared by direct compression method. Contain combination of the disintegrating agent and gives less disintegration time.
Material
MO seed kernel powder is obtained from Nashik District, Maharashtra, India
Method
Isolation of moringa powder: Mature seeds of M. oleifera were chosen from dry cracked fruits. The plucked fruits were cracked to obtain the seeds which were air-dried for 2 days. The shells surrounding the seed kernels were removed using a knife, and the kernels were powdered using a laboratory mortar and pestle and sieved using a sieve with a pore size of 2.5 mm2 to obtain a fine powder. The powder was stored in a sterile bottle at room temperature in a dark place.
Preparation of tablet blend: The drug and excipient were passed through sieve (40) to ensure better mixing, mannitol was used as a direct compressible vehicle. Super disintegrant like cross povidone and MCC were used in different proportion. all the ingredients without magnesium stearate and talc were mixed uniformly followed by addition of magnesium stearate and talc.the prepared powder blend was evaluated for various parameters like Bulk density, Tapped density, Angle of repose, Carr’s index, and Hausner’s ratio.
Preparation of dispersible tablet: Dispersible tablets containing 60 mg of MO seed powder and 60 mg Alum were prepared by direct compression method and various formulae used in the study. After evaluation of powder blend the tablet were compressed with a tablet punching machine using 10.5 mm biconvex punch.
Factorial design
Based on the result obtained with preliminary formulations, 23 factorial design was applied in the present study, In this design 3 factors were evaluated, each at 2 levels and experimental trials were done at all 8 possible combinations. The concentration of drug, CPV, MCC were selected as independent variable because this variable has high impact on dependent variable. Hardness, disintegration time was categorized as dependent variable. The resulting data were fitted into Design Expert Software and analysed statistically using analysis of variance.
From all trial batches following concentration of ingredient was decided for further study (Table 1).
| Ingredients | Concentration (% w/w) |
|---|---|
| Moringa oleifera | 16-20 |
| Alum | 16-20 |
| Cross povidone | 2-5 |
| MCC | 20-90 |
| Mannitol | 10-90 |
| Mg. stearate | 0.25-5 |
| Talc | 5-30 |
| Aerosil | 0.15-0.75 |
Table 1. Formula for dispersible tablet.
FTIR spectra of Moringa oleifera
FTIR spectroscopy was performed on FTIR spectrophotometer and it was used to study and find out whether there are many interactions between the drugs and excipients used in the formulation (Table 2).
| Sr. No | Binary mixture Condition: 40°C/75% RH, closed container, 1 month |
Ratio |
|---|---|---|
| 1 | MO+Alum | 1:1 |
| 2. | MO+CPV | 1:1 |
| 3. | MO+MCC | 1:1 |
| 4. | MO+Mannitol | 1:1 |
| 5. | MO+Mg. Stearate | 1:1 |
| 6. | MO+Talc | 1:1 |
| 7. | MO+Aerosil | 1:1 |
Table 2. Drug excipients compatibility study (binary mixture).
Determination of concentration of alum for flocculation
In 1 lit of untreated water alum was added sequentially from 10 mg to 70 mg in labelled beaker of the water [7]. The clearity of the water was observed by eye.
Determination of concentration of MO for flocculation
Moringa seed powder was added to the 1 litre water beaker containing untreated water [8]. The seed powder was added in different seven beaker of water. The degree of flocculation was studied after flocculation occurs for suspended particle present in water.
Study of effect of stirring for clearity of water
Stirring rate was studied by using magnetic stirrer at 5 min, 10 min, 15 min so on upto 25 min.
Precompression study of powder blend
Bulk density: The sample (equivalent to 25 g) was accurately weighed and filled in a 100 ml graduated cylinder and the unsettled volume of the powder, Vo was noted. The bulk density was calculated by the formula.
Bulk density (ρo)=M/Vo (1)
Where,
M=mass of powder taken; Vo=Apparent unstirred volume;
Tapped density: The tapped density was determined by mechanically tapping the measuring cylinder and the volume was noted (6).
Tapped density (ρt)=M/Vt (2)
Where,
ρt=tapped density; M=weight of granules; Vt=tapped volume of granules in cm3
Angle of repose: Angle of repose is defined as the maximum angle possible between the surface of pile of powder and horizontal plane. The angle of repose was determined by the funnel method. The accurately weighed powder was taken in a funnel. The height of the funnel was adjusted in such a way that the tip of the funnel just touched the apex of the heap of the powder. The powder was allowed to flow through the funnel freely onto the surface. The diameter of the powder cone was measured. The angle of repose was calculated by substituting the values of the base radius ‘R’ and pile height ‘H’ in the following equation.
Tan̬̉=H/R (3)
Where,
H=Pile height; R=Radius of Pile Therefore;
Therefore; Ãâè=tan–1 H/R
Compressibility index: The bulk volume and tapped volume was measured and compressibility index was calculated using the formula,
Compressibility index =100 (Vo-Vf)/Vo× (4)
Where,
Vo=Bulk volume; Vf=Tapped volume
Hausner’s ratio: Tapped volume and bulk volume were measured and the Hausner’s ratio was calculated using the formula.
Hausner’s ratio=Vo/Vf (5)
Where, Vo=Bulk volume; Vf=Tapped volume; Lower Hausner’s ratio=better flowability; Higher Hausner’s ratio=poor flowability
Evaluation of dispersible tablets (Post compression parameter)
Physical parameters
Thickness; The thickness of the tablets was determined using a Vernier caliper. Five tablets from each type of formulation were used and average values were calculated. It is expressed in mm.
Hardness: The resistance of tablets to shipping, breakage, under conditions of storage, transportation and handling before usage depends on its hardness. For each formulation, the hardness of 6 tablets was determined using the Monsanto hardness tester. The tablet was held along its oblong axis in between the two jaws of the tester. At this point, reading should be zero kg/cm2. Then constant force was applied by rotating the knob until the tablet fractured. The value at this point was note.
Friability: Friability is the measure of tablet strength. Roche Friabilator was used for testing the friability using the following procedure. This test subjects number of tablets to the combined effect of shock abrasion by utilizing a plastic chamber which revolves at a speed of 25 rpm, dropping the tablets to a distance of 6 inches in each revolution. A sample of preweighed 6 gm tablets were placed in Roche friabilator which was then operated for 100 revolutions i.e. 4 minutes. The tablets were then dusted and reweighed. A loss of less than 1% in weight in generally considered acceptable. Percent friability (%F) was calculated as follows.
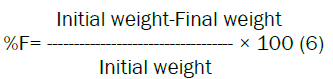
Weight variation test: To find out weight variation, 20 tablets of each type of formulation were weighed individually using an electronic balance, average weight was calculated and individual tablet weight was then compared with average value to find the deviation in weight.
Preparation of water sample: To study the water quality or for performing evaluation of water for turbidity, TDS, PH, Conductance, microbial count. Water sample were prepared by adding 10 gm of soil which is fine in nature is added to 1 litre of water that water was used for further evaluation [9,10].
Evaluation of water: pH of each formulation was determined by using digital pH meter. The pH meter was calibrated using pH 4 and pH 7 buffer by using standard buffer tablet.
Conductivity: Take 100 ml of sample in closed bottle and read the conductivity at 20ºC on suitably calibrated conductivity meter.
TDS: TDS values are useful to determine whether water is suitable for drinking purpose, agriculture and industrial purposes. The TDS values in the present study ranged within 305 mg/lt to 729 mg/lt.
Hardness of water: Water with Hardness above 200 mg/lit. May cause scale deposition in the distribution system and results in excessive soap consumption and subsequent scur formation [11,12]. Soft water with hardness of less than 100 mg/lit may have lower buffer capacity and more corrosive to water pipes.
Turbidity: Turbidity of water was measured by using calibrated turbidity meter. Instrument was calibrated prior to use. The higher the intensity of light higher the turbidity.
Microbial count: By MPN test (Most Probable Number)
Presumptive test: Presumptive test involves the primary presumption for the presence of Gram negative coliform bacteria in the samples demonstrated by the appearance of gas in the lactose fermentation broth. For the presumptive test procedure 15 sets of test tubes containing lactose fermentation broth required for each sample under analysis. Each test tube contained 10 ml of fermentation broth and inoculated with the water sample in a sequential order of 10 ml in five of each 2X lactose fermentation broth, 1 ml in five of each 1x lactose fermentation broth and lastly 0.1 ml in five of each 10 ml 1X lactose fermentation broth. All the test tubes were incorporated with Derhum tubes for detection of gas formation by Gram negative coliform bacteria. Test tubes were incubated with half circled screw caps at 37̢̮̉ for 48 hours. This procedure was followed for all of the 75 samples individually
Confirmed test: Positive samples with the production of gas in the lactose fermentation broth were selected for the confirmed test procedures to detect the indicator bacteria of fecal origin Escherichia coli. EMB media was used to differentiate other Gram negative coliform bacteria from the Escherichia coli by the production of green metallic sheen in the media. The presence of green metallic sheen in EMB confirms the presence the indicator bacteria E. coli. One loopful sample from the positive test tubes was inoculated on EMB by streaking and incubated at 37ºC for 24 hours and then observed for the production of green metallic sheen.
Completed test: From the positive EMB plates showing green metallic sheen colonies of E. coli, the isolated colonies were inoculated into LFB 1X media containing Derhum tube to re-confirm the positive lactose fermentation. From the same colony, indicator organism (E. coli) was observed microscopically for their Gram reactions. This was the final stage of the MPN method where in the decision of water quality as potable or non-potable, could be made after confirmation and completion of the study. Finally, the standard biochemical tests were performed to confirm the identification of all the pathogenic isolates found in all 75 types of drinking water samples by the previously described methods.
Antimicrobial study of Moringa oleifera
Agar disk-diffusion method: In this well-known procedure, agar plates are inoculated with a standardized inoculum of the test microorganism. Then, filter paper discs (about 6 mm in diameter), containing the test compound at a desired concentration, are placed on the agar surface (Table 3). The Petri dishes are incubated under suitable conditions. Generally, antimicrobial agent diffuses into the agar and inhibits germination and growth of the test microorganism and then the diameters of inhibition growth zones are measured [13-17]. According to literature MO seeds are having anti-bacterial and antifungal activity (Figure 1).
IR Spectrum of the MO was recorded and spectrum shows distinguished peak at 3420 cm-1,2923 cm-1,2852 cm-1,1750 cm-1, 1630 cm-1 and 1587 cm-1 to O-H stretching, N-H stretching, C-H, CH2, C=O, and CN stretching. It was observed that peak comply with recorded literature (Table 3).
| Peak (cm-1) | Interpretation |
|---|---|
| 3420 | O-H stretching |
| 2923 | N-H stretching |
| 2852 | C-H |
| 1750 | CH2 |
| 1630 | C=O |
| 1587 | CN stretching |
Table 3. IR ranges observed in All IR Spectra of compatibility study [14].
Compatibility study
All IR spectra of Compatibility study showed functional group of Moringa oliefera. Drug was found to be compatible with all selected components of formulation in binary study (Table 4).
| Sr. No. | Concentration of alum | Effect |
|---|---|---|
| 1 | 10 | |
| 2 | 20 | |
| 3. | 30 | |
| 4. | 40 | Decreased turbidity |
| 5. | 50 | |
| 6. | 60 | |
| 7. | 70 |
Table 4. Determination of concentration of alum for flocculation.
From observation it was concluded that concentration more than 50 mg decreased the turbidity from the water.
Determination of concentration of MO for flocculation
Moringa seed kernel contain protein due to presence of protein in the seed. Particulate matter forms a flocs with cationic
charge present on the protein. Due to this mechanism of floc formation purification of the water occurs (Tables 5-7).
| Sr. No. | Concentration of Moringa | Effect |
|---|---|---|
| 1 | 10 | |
| 2 | 20 | |
| 3. | 30 | |
| 4. | 40 | Increase flocculation |
| 5. | 50 | |
| 6. | 60 | |
| 7. | 70 |
Table 5. Concentration of MO for flocculation.
| Batch | Mg of MO/1000 mL water | stirring rate (min) |
|---|---|---|
| 80 mg | 5 | |
| 80 mg | 10 | |
| 80 mg | 15 | |
| 80 mg | 20 | |
| 80 mg | 25 |
Table 6. Effect of stirring for clearity of water.
| Batch No. |
Angle of repose (º) |
Bulk density gm/mL |
Tapped density (gm/mL) |
Carr’s Index | Hausner’s ratio |
|---|---|---|---|---|---|
| F1 | 23.29 ± 0.89 | 0.619 ± 0.02 | 0.699 ± 0.04 | 12.76 ± 0.23 | 1.129 ± 0.04 |
| F2 | 24.61 ± 1.18 | 0.606 ±0.01 | 0.714 ± 0.02 | 15.15 ± 0.46 | 1.178 ± 0.006 |
| F3 | 26.41 ± 0.49 | 0.625 ± 0.01 | 0.741 ± 0.02 | 15.63 ± 0.48 | 1.185 ± 0.007 |
| F4 | 25.10 ± 0.51 | 0.626 ± 0.03 | 0.691 ± 0.03 | 9.39 ± 0.49 | 1.103 ± 0.006 |
| F5 | 24.31 ± 0.85 | 0.555 ± 0.01 | 0.625 ± 0.01 | 11.60 ± 1.13 | 1.125 ± 0.003 |
| F6 | 23.44 ± 1.56 | 0.601 ± 0.03 | 0.661 ± 0.04 | 9.02 ± 0.58 | 1.098 ± 0.007 |
| F7 | 22.55 ± 0.85 | 0.583 ± 0.02 | 0.682 ± 0.03 | 14.57 ± 0.64 | 1.170 ± 0.008 |
| F8 | 25.02 ± 0.76 | 0.571 ± 0.01 | 0.645 ± 0.02 | 11.92 ± 0.90 | 1.129 ± 0.004 |
Table 7. Precompression study of powder blend.
Study of effect of stirring for clearity of water
From above result it was concluded that if the stirring rate is increase more than 5 min turbidity in the water is get increased may be because of there is disturbance in the floc. Formation from the result it was concluded that more than 5 min stirring increases the turbidity in water (Figure 2).
Precompression study of powder blend
Bulk density depends upon particle size, shape, and tendency of particles to adhere together. The values for BD and TD are shown in Table 7 these were found to range from 0.555 ± 0.03 to 0.626 ± 0.025 and 0.625 ± 0.001 to.741 ± 0.02 for powder blend respectively.
The angle of repose and Carr’s Index of powder blend was found in the range of 22.55 ± 0.08 to 26.90 ± 0.07 and 9.02 ± 0.58 to 15.63 ± 1.31 respectively. Hausner’s ratio was found in the range of 1.098 ± 0.007 to 1.178 ± 0.006.6 (Tables 8 and 9).
| Formulations | Hardness (kg/cm2 ± SD) |
Thickness (mm ± SD) |
% Friability (% ± SD) |
Wt. variation (mg ± SD) |
DT (Sec) |
|---|---|---|---|---|---|
| F1 | 2.510 ± 0.0040 | 5.2 ± 0.15 | 0.2 ± 0.305 | 302.00 ± 10 | 57.40 ± 2.30 |
| F2 | 2.510 ± 0.0060 | 5.1 ± 0.43 | 0.32 ± 0.159 | 298.00 ± 15 | 57.21 ± 2.00 |
| F3 | 2.512 ± 0.0047 | 5.5 ± 0.05 | 0.28 ± 0.102 | 298.00 ± 10 | 57.54 ± 3.05 |
| F4 | 2.507 ± 0.0055 | 5.2 ± 0.3 | 0.85 ± 0.133 | 298.00 ± 05 | 58.26 ± 2.00 |
| F5 | 2.509 ± 0.0040 | 5.4 ± 0.4 | 2.39 ± 0.189 | 300.00 ± 10 | 56.96 ± 2.5 |
| F6 | 2.500 ± 0.0032 | 5.9 ± 0.21 | 1.01 ± 0.253 | 302.00 ± 20 | 55.34 ± 2.8 |
| F7 | 2.508 ± 0.0030 | 5.5 ± 0.32 | 0.85 ± 0.165 | 297.00 ± 10 | 60.25 ± 3.2 |
| F8 | 2.509 ± 0.0040 | 5.5 ± 0.05 | 0.79 ± 0.220 | 296.00 ± 10 | 57.29 ± 1015 |
Table 8. Compression properties of dispersible tablet.
| Sr. No. | Batch | PH |
|---|---|---|
| 1. | F1 | 7.51 |
| 2. | F2 | 7.52 |
| 3. | F3 | 7.51 |
| 4. | F4 | 7.23 |
| 5. | F5 | 7.51 |
| 6. | F6 | 7.21 |
| 7. | F7 | 7.44 |
| 8. | F8 | 7.51 |
Table 9. Evaluation of water.
Post compression properties of dispersible tablet
Evaluation of water: Ph of water
Normal range of PH for the drinking water was 6.5 to 8.5 according to regulation. Observed PH of the formulation was found in between 7.2 to 7.5 means the formulation passes the limit and gives proper PH of water (Table 10).
| Parameter | Observed range | Reported range | Unit |
|---|---|---|---|
| pH | 7.25 | 6.5 | 8.5 |
| Conductance | 357 | - | μmho/cm |
| turbidity | BDL (DL:0.2) | Max.1 | NTU |
| TDS | 200 | Max.500 | Mg/L |
| Total Hardness | 130 | Max.200 | Mg/L |
| Aluminium content | BDL(DL:0.025) | Max.0.03 | Mg/L |
| Total Alkalinity | 80 | Max.200 | Mg/L |
Table 10. Results for optimized batch.
From all performed test, slightly alkaline pH was most desirable because this range is most comfortable to the human eye. Conductance was measured to calculate what was dissolved in water. Salinity of water was increased with increased in conductance, this affect the life of aquatic animals. TDS was used as an indication of aesthetic characteristics of drinking water and as an aggregate indicator of the presence of a broad array of chemical contaminants. Hardness was calculated for studied amount of calcium and magnesium present in the water. Increased intake of Magnesium salts may cause a change in bowel habits (diarrhea). Drinking-water in which both magnesium and sulfate are present in high concentrations (~250 mg/l each) can have a laxative effect may cause cardiovascular diseases, cancer. The Aluminums content more than limit may cause Alzheimer’s disease. The risk of Alzheimer's disease was 1.5 times higher in districts where the mean aluminum concentration exceeded 0.11 mg/l than in districts where concentrations were less than 0.01 mg/l. Above result it was concluded that all the evaluation parameter are within limit and the aluminium content was also not increase by addition of the aluminium sulphate in the water. According to this result the water can be drinkable by the individual during travelling, trekking etc. (Tables 11 and 12).
| No. of tubes giving positive reaction out of | MPN Index per 100 ml | No. of tubes giving positive reaction out of | MPN index per 100 ml | ||||
|---|---|---|---|---|---|---|---|
| 5 of 10 ml Each | 5 of 1 ml Each | 5 of 0.1 ml Each | 5 of 10 ml Each | 5 of 1 ml Each | 5 of 0.1 ml Each | ||
| 0 | 0 | 0 | <2 | 4 | 2 | 1 | 26 |
| 0 | 0 | 1 | 2 | 4 | 3 | 0 | 27 |
| 0 | 1 | 0 | 2 | 4 | 3 | 1 | 33 |
| 0 | 2 | 0 | 4 | 4 | 4 | 0 | 34 |
| 1 | 0 | 0 | 2 | 5 | 0 | 0 | 23 |
| 1 | 0 | 1 | 4 | 5 | 0 | 1 | 31 |
| 1 | 1 | 0 | 4 | 5 | 0 | 2 | 43 |
| 1 | 1 | 1 | 6 | 5 | 1 | 0 | 33 |
| 1 | 2 | 0 | 6 | 5 | 1 | 1 | 46 |
| 2 | 0 | 0 | 5 | 5 | 1 | 2 | 63 |
| 2 | 0 | 1 | 7 | 5 | 2 | 0 | 49 |
| 2 | 1 | 0 | 7 | 5 | 2 | 1 | 70 |
| 2 | 1 | 1 | 9 | 5 | 2 | 2 | 94 |
| 2 | 2 | 0 | 9 | 5 | 3 | 0 | 79 |
| 2 | 3 | 0 | 12 | 5 | 3 | 1 | 110 |
| 3 | 0 | 0 | 8 | 5 | 3 | 2 | 140 |
| 3 | 0 | 1 | 11 | 5 | 3 | 3 | 180 |
| 3 | 1 | 0 | 11 | 5 | 4 | 0 | 130 |
| 3 | 1 | 1 | 14 | 5 | 4 | 1 | 170 |
| 3 | 3 | 0 | 17 | 5 | 4 | 4 | 350 |
| 4 | 0 | 0 | 13 | 5 | 5 | 0 | 240 |
| 4 | 0 | 1 | 17 | 5 | 5 | 1 | 350 |
| 4 | 1 | 0 | 17 | 5 | 5 | 2 | 540 |
| 4 | 1 | 1 | 21 | 5 | 5 | 3 | 920 |
| 4 | 1 | 2 | 26 | 5 | 5 | 4 | 1600 |
| 4 | 2 | 0 | 22 | 5 | 5 | 5 | ≥1600 |
Table 11. MPN test.
| Concentration of media | Treated water | Untreated water |
|---|---|---|
| 10 mL | 4 | 5 |
| 1 mL | 1 | 5 |
| 0.1 mL | 0 | 1 |
Table 12. Observations of MNP test.
From the observation after incubation of the test tube gas production and yellow colour was recorded as positive tube. In 10 mL concentration of media shows 4 positive test tube, 1 mL concentration of media shows 1 positive test tube, and 0.1 mL concentration shows shows 0 positive test tube. MPN Index per 100 mL was found to be 17.
Antimicrobial test for Moringa oleifera
Antimicrobial avtivity of moringa was studied at 10 mg moringa/1 ml water sample against E-coli strain. After 24 hrs. At 36ºC it shows 3.6 cm zone of inhibition for E-coli. From this result it was concluded that it shows antibacterial activity (Figure 3).
The active ingredient present in marketed water purification tablet includes iodine, chlorine et al. These ingredient having disinfectant action, but them unable to form flocs in the water. So it cannot remove particulate water from the water. So, objective of the study was to developed tablet formulation for water purification.
These tablet contain Alum and MO powder as an active ingredient which gives clear water and MO seed powder having antimicrobial activity. The antimicrobial activity of the MO seed powder was studied with the help of Agar disk-diffusion method and gave 3.6 cm of zone of inhibition.
The tablet for water purification Is prepared by direct compression Various batches are formulated by changing the concentration of drug and disintegrant. This data is used for factorial design set up 23 factorial design is used. DT and Hardness is consider as a dependent variable at two different levels. Tablet formulation is evaluated by precompression parameter including carr’s index, Angle of repose, Hausners ratio, and post compression parameters includes Hardness, Friability, DT.
The angle of repose is between 22.55-26.41, Carr’s index is found to be 9.02-15.63, and Hausner’s ratio is 1.09-1.18. All the precompression parameter are with in limit. DT and hardness are consider as dependent variable, optimized batch having DT 55-60 sec. and hardness is 2.5 kg/cm2.
This formulated tablet significantly decreased Turbidity and coliform, the water which is purified by using this tablet is evaluated for TDS, Turbidity, PH, Conductance and Aluminium content. TDS of treated water is 200 mg/l turbidity is also less than 1 NTU, Aluminium content is not increased by addition of aluminium sulphate.