e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
Melike Yılmaz*, Cüneyt Toprak, Gökay Gün, Mahmut Özbek
Research and Development Centre, World Medicine Ä°laç San. ve Ticaret A.Ş., Ä°stanbul, Turkey
Received date: 28/01/2021; Accepted date: 13/02/2021; Published date: 22/02/2021
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
Ivermectin has antiviral activity which may play an important role in several essential biological processes. Hence, it could be a potential candidate in the treatment of different kinds of viruses including COVID-19. Objective of this study was to formulate Ivermectin 3 mg Tablet to improve particle size distribution homogeniously. Three lab-scale trials and pilot study were performed with different batch size equipment to provide ideal filling ratio providing homogeniously blending. The manufacturing process was optimised in Trial-3 and then proses validation working was confirmed. Acceptable value of content uniformity of Trial-3 and pilot study was calculated as 3.93% and 3.71%, respectively. This study was indicated the importance of selecting suitable equipment to reach ideal blending in low dose tablet formulation by direct compression method.
Ivermectin 3 mg Tablet; Blend Homogenity; Geometric dilution
Ivermectin is formed a mixture of component H2B1a and component H2B1b as shown in Figure 1. It has been used to treat many infectious diseases in mammals. It posseses antiparasitic and antiviral effects and leads to immunomodulation in the host. Ivermectin was found in late 1970s and it was firstly tried for animal studies in 1981[1]. Ivermectin is approved by FDA (Food Drug Administration) and inhibites in vitro replication of SARS-CoV-2. Moreover, it has an established safety profile for human use. Recent studies have shown that the standard low-dose ivermectin has considerable safety than high dose treatment [2,3]. Therefore, 3 mg tablet dose was formulated by direct compression method to provide active substance of homogenious distribution in the tablet.
Figure 1. The molecular structure of Ivermectin [2].
Ivermectin molecule is defined crystalline powder which seems a white to yellowish-white, Its physical properties following as; It is nonhygroscopic and melting point of it is about 155°C. In addition, it is soluble in 95% ethanol, freely soluble in methanol and insoluble in water. 3 mg and 6 mg tablets which are available in the market called Stromectol (Merck, Canada). It contains the following excipients Microcrystalline Cellulose, Pregelatinized Starch, Magnesium Stearate, Butylated Hydroxyanisole, and Citric Acid Anhydrous Powder.
Pharmaceutical development of Ivermectin 3 mg uncoated tablet is an established pharmaceutical form and its development is adequately described according to the relevant European guidelines. The selection of excipients is justified in accordance with manufacturing method and their functions explained in Table 1. Ivermectin has very limited solubility in water. Ivermectin is defined BCS (Biopharmaceutical classification system) class-II drug, which has high permeability and low water solubility (0.005mg/ml) [4] and therefore, its particle size distribution is very important. The particle size distribution of the active substance has been optimised. The formulation was developed as an uncoated tablet with a drug release similar to that of the reference product called Stromectol.
| Excipient name | Spesification | Function |
|---|---|---|
| Avicel PH 102 | d10 15-55 µm d50 80-140 µm d90 170-283 µm |
Filler |
| Acdisol SD 600 | Air jet particle size, wt% +200 Mesh (+75 µm) Air jet particle size, wt% +325 Mesh (+44 µm) |
Disintegrant |
Table 1. Particle size distribution of filler and disintegrant in the formulation.
Blending is a direct compression process so as to achieve a homogenous product at low dose drug for manufacturing of oral drug delivery systems. Uniformity of active substances plays a key role because it will effect drug dissolution, absorption, bioavailability. When low load drugs formulations are developed, blend homogeneity factors should be considered such as particle size, size distribution, density of the individual components and equipment size [5].
In this study, we summarized the formulation of Ivermectin 3 mg Tablet by direct compression of geometric dilution method and showed the importance of the effects of the excipient particle size, the blending techniques and the content uniformity of low-dose Ivermectin as a drug [6].
Ivermectin was obtained from HISUN (China) as active substances. Avicel PH 102® (FMC, Switzerland), Aerosil 200 (Evonik, Germany), Acdisol SD 600 (Dupont, Ireland) and Magnesium Stearate (Peter Greven, United Kingdom) were used as inactive substances. All materials are suitable for geometric blending process and particle size distribution of filler and disintegrant are indicated in Table 2.
Preparation of powder mixtures by geometric dilution as following formulation in Table 2. In addition, all tablet formulation theoretically was contained 3 mg of active substance.
| Ingredients | Function | Trial 1 | Trial 2 | Trial 3 | Proses validation series |
|---|---|---|---|---|---|
| Ivermectin | Active Substance | 3 mg | 3 mg | 3 mg | 3 mg |
| Avicel PH 102® (MCC 102) | Filler | - | - | - | - |
| Aerosil 200 | Glidan | - | - | - | - |
| Acdisol SD 600 | Disintegrant | - | - | - | - |
| Magnesium Sterate | Lubricant | - | - | - | - |
Table 2. Formulation of Ivermectin 3 mg Tablet.
The flow ability parameters such as bulk density, tap density compressibility index and Hausner’s ratio was evaluated.
Bulk density is defined as a measure to describe packing materials of powders. Basic FGF treated samples.
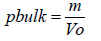
Where,
m= Mass of the blend
V0=Untapped volume
Tapped Density:
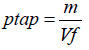
Where,
m= Mass of the blend`
V f=Tapped volume
Compressibility Index or Carr’s Index is defined as features of a powder to be compressed.
Carr’s index (CI) was determined by measuring the initial volume (pbulk) and final volume (ptap) after five hundred tapings of a sample of ivermectin bulk in a measuring 100 ml of cylinder by using Tapped Density machine (Erweka, Germany).
Carr’s index (CI) and Hausner Ratio (HR) were calculated using as following equations;
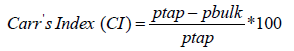
Hausner Ratio (HR) shows the flow properties of the powder. It defines as the ratio of tapped density to the bulk density of the powder.
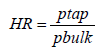
Bulk density (pbulk): 0.334 (g/ml)
Tapped density (ptap.): 0.392 (g/ml)
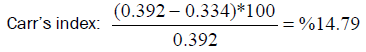
Hausner Ratio (HR):
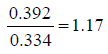
Carr’s index value and Hausner Ratio was found as %14.79 and 1.17, respectively. According to Flow character table, the flow of bulk was determined as good (Table 3).
| Flow character | Carr’s index (% Compressibility) | Hausner’s ratio |
|---|---|---|
| Excellent | 5 – 15 | 1.00 – 1.11 |
| Good | 12 – 16 | 1.12 – 1.18 |
| Fair | 18 – 21 | 1.19 – 1.25 |
| Possible | 23 – 28 | 1.26 – 1.34 |
| Poor | 28 – 35 | 1.35 – 1.45 |
| Very poor | 35 – 38 | 1.46 – 1.59 |
| Extremely poor | >40 | >1.60 |
Table 3. Compressibility and Hausner’s Ratio according to flow character.
Lab-scale studies
Geometric blending is a common technique of direct compression to blend small amounts of Active Pharmaceutical Ingredient (API). All formulations were performed by geometric blending technique.
Trial 1, Trial 2 and Trial 3 were performed for an amount of 520 g, 1020 g and 1020 g as a batch size, respectively. In Trial 1, Active substance was sifted from a sieve with apertures 0,5 mm and then active substance and excipientes were added step by step by geometric dilution method. Afterthat, all steps except of adding lubricant stage were mixed at 60 rpm for 10 min by 7,2 L of cubic mixer (Erweka, Munich, Germany) as shown in Figure 1.
In Trial 2, Active substance and Acdisol SD 600 were sifted from a sieve with apertures 0,6 mm and then excipientes were added step by step by geometric dilution method. Afterthat, Stage 1 and Stage 2 were mixed at 60 rpm for 10 min by 3,2 L of cubic mixer (Erweka, Munich, Germany). The other stages except of adding lubricant stage were were mixed at 60 rpm for 10 min by 7,2 L of cubic mixer (Erweka, Munich, Germany). All proses was summarized in Figure 2.
In Trial 3, It was decided to decrease of sieve of active substance and disintegrant and time of mixing in the all stages to provide homogeniosly particle size distrubution. Ivermectin and Acdisol SD 600 were sifted from a sieve with apertures 0,4 mm and then excipientes were added step by step by geometric dilution method. Afterthat, Stage 1 and Stage 2 were mixed at 60 rpm for 15 min by 3,2 L of cubic mixer (Erweka, Munich, Germany). The other stages except of adding of lubricant were were mixed at 60 rpm for 15 min by 7,2 L of cubic mixer (Erweka, Munich, Germany). All proses was summarized in Figures 3-5.`
Finally, each batch was lubricated with magnesium stearate by additional mixing at 60 rpm for 3 min.
Pilot study
Manufacturing process of pilot study was smilar to Trial 3. Pilot study was performed for an amount of 39.1 kg as a batch size. Ivermectin and Acdisol SD 600 were sifted from a sieve with apertures 0.4 mm and then related excipientes were added step by step by geometric dilution method. Afterthat, Stage 3 was mixed at 12 rpm for 15 min by 25 L of container mixer (HSD).`
Stage 4 was mixed at 12 rpm for 15 min by using 50 L container mixer [7]. The mixing in Stage in Stage 5 was mixed at 12 rpm for 15 min using 100L container and stage 6 and the other stages mixed with 600 L in container mixer as summarized in Figure 6.
Filling ratio of equipments was calculated according to bulk density result which was accepted as 0.334 g/ml in all stage of both trials and pilot study (Figure 7, Tables 4 and 5).
| Trial 1 Batch Size:520 g |
Trial 2 Batch Size:1020 g |
Trial 3 Batch , Size:1020 g |
Pilot study Batch Size:39,1 kg |
|
|---|---|---|---|---|
| Used equipment | 5,8 L of cube mixer | 2,7 L of cube mixer 5,8 L of cube mixer |
2,7 L of cube mixer 5,8 L of cube mixer |
25 L container mixer 50 L container mixer 100 L container mixer 600 L container mixer |
Table 4. Used Equipment according to Filling ratio.
Accepted value (AV) calculation for Pilot study
X=Average
k=2.4
S=Standart Deviation=1.31
X= %97.93 If X < 98.5%, then
AV=98.5 – X + ks
AV=98.5-97.93+(2.4*1.31)
AV=3.71
| Conditions | Values | |
|---|---|---|
| If 98.5%<X<101.5, then | M=X | AV=ks |
| If X<98.5%, then | M=98.5% | AV=98.5 – X + ks |
| If X>101.5, then | M=101.5% | AV=X– 101.5 + ks |
Table 5. Accepted value (AV) calculation conditions [2].
Pharmacope limit (HPLC Eur. Ph. 2.2.29 and Eur. Ph. 2.9.40) is that Acceptance value of 10 units is less than or equal to 15.0% [2].
When trials were completed, results of trials were summarized in Table 6. Results in Table 6 were obtained that taking ten different tablet samples for each trials were individually analysed of tablet content that resulted in Average, Standart deviation, Relative Standart Deviation and Accepted value. It was seen that when all results were examined, variation in Trial-3 was lower than Trial-1 and Trial-2 [8]. When similar manufacturing method of pilot study like Trial-3 was performed, it was obtained successful result as shown in Figure 8.
Manufacturing method of Trial 1, Trial 2 and Trail 3 were optimised and Ideal container mixer which will be used in pilot study was selected according to using raw materials on calculation of bulk density. In pilot study, Filling ratio of 25 L, 50 L, 100L and 600L was calculated as 56.23%, 60.61%, 63.0% and 21.72% [9,10]. Filling ratio for ideal bleding process should be between 40% and 70%. The final mixing steps were made in the 600 L container mixer, but the appropriate result was obtained in mixing in the 600 L container, as proper homogeneity was achieved in the previous mixing orders.
Hence, used in different size of container mixer according to filling ratio had the lowest standard deviation compared to all carrier formulations. Table 6 demonstrates that the drug distribution for the different types of blends requires chosing ideal equipment, mixing in longer duration to obtain more homogenous blend (Table 6).
| Uniformity of dosage units (Content uniformity) 3 mg |
Results (%) |
|||
|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 3 | Pilot study | |
| Sample-1 | 107.84 | 96.58 | 99.84 | 98.51 |
| Sample-2 | 96.37 | 96.97 | 102.51 | 98.05 |
| Sample-3 | 92.34 | 108.63 | 97.42 | 96.95 |
| Sample-4 | 115.23 | 93.40 | 100.58 | 98.27 |
| Sample-5 | 126.97 | 102.58 | 97.68 | 97.99 |
| Sample-6 | 117.35 | 100.22 | 98.2 | 98.31 |
| Sample-7 | 110.24 | 103.78 | 100.97 | 96.94 |
| Sample-8 | 106.8 | 91.40 | 99.30 | 98.27 |
| Sample-9 | 108.86 | 96.35 | 100.54 | 100.53 |
| Sample-10 | 108.45 | 97.68 | 101.57 | 95.50 |
| Average | 109.04 | 98.76 | 99.92 | 97.93 |
| Standard Deviation | 9.87 | 5.13 | 1.64 | 1.31 |
| Relative Standard Deviation (RSD) | 9.05 | 5.20 | 1.64 | 1.33 |
| Accepted value % (AV) | 33.27 | 12.32 | 3.93 | 3.71 |
Table 6. Content uniformity results of Ivermectin 3 mg Tablet.
Ivermectin is an antiparasitic drug used in treating cabies, parasitic worms and head lice. It also acts against other intestinal nematodes. Lab-scale studies to pilot study were performed using geometric blend technique in low dose drug model. Manufacturing method was optimised and then ideal equipment was selected according to bulk density of related excipients. Content uniformity of tablets in Trial 3 and pilot study were analysed and their accepted value was calculated according to conditions which is refer to USP pharmacope. There is variation in Trial-1 and Trial 2 because of equipment capacity which was not ideal filling ratio of powder inside equipment. However, Blend homogenity in Trial-3 and Pilot study was provided so that the filling ratio was an ideal. Their Accepted value was found smaller than 15 which were 3.71.
It can be concluded that an increase in blending time, a decrease in sieve size of API and container mixer size which is used in manufacturing process with geometric addition showed considerable uniformity improvements for manufacturing of Ivermectin 3 mg Tablet. This will be improved drug dissolution, absorption and bioavailability. Moreover, It has been observed that the mixing volume of the equipment is critical, depending on the bulk density of the powder, for homogeneous mixing in this product.
This work was supported by World Medicine İlaç San. ve Tic. A. Ş.