ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Meenakshi S1, Sukhada Mohandas2, Riaz Mahmood3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Agrobacterium-mediated transformation was developed for tomato plant (cv. Arka vikas) using Antimicrobial peptide gene under the control of CaMV 35S promoter in pCAMBIA 2301 vector. Protocol was optimised by various parameters influencing transformation. Hypocotyledonary explants where treated with Agrobacterium 0.4 O.D 600 for 2 min and selected on kanamycin 100 mg l-1. 59 shoots regenerated initially on MS media containing BAP – 2.0 mg l-1 and TDZ – 1.0 mg l-1. 13 plants were finally survived on hardening and screened for the presence of gene by PCR. 360 bp of gene amplification confirmed the presence of gene in 8 plants. Transformation frequency of 11 % was observed.
KEY WORDS |
| Transgenic tomato, Optical density, Duration, Co-cultivation, Agrobacterium transformation, |
I. INTRODUCTION AND BACKGROUND |
| Tomato (Lycopersicon esculentum Mill.) is the major vegetable crop grown worldwide in production and consumption. India being the second highest in production of tomato according to FAOSTAT in 2012. Tomato has been widely used as a model crop in genetic manipulation experiments due to its ease of transformation and small genome (Arumuganathan et al, 1991). Various factors involve in efficient Agrobacterium transformation such as, Arobacterium cell density (Murray et al., 1998), regeneration and co-cultivation conditions (Hu and Phillips, 2001) and cell competence after wounding (Murray et al., 1998). McCormick, et al. (1986) first reported on tomato transformation and ever since, numerous publications on transformation of various tomato cultivars (Sree Vidya et al., 2000; Hu and Phillips, 2001; Park et al., 2003; Raj et al., 2005; Sun et al., 2006 and Saker et al., 2007). Development of highly regenerative transformation protocol is essential in Agrobacterium transformation. The present study was undertaken to develop an efficient procedure for the production of fungal-resistant transgenic tomato plants expressing Antimicrobial peptide gene. |
II. MATERIALS AND METHODS |
• Plant tissue |
| Seeds of tomato (Solanum lycopersicum L) cultivar Arka vikas were surface sterilised in 4% (v/v) sodium hypochlorite solution, with 0.01% of Tween – 20 for 20 mins followed by three washes with sterile distilled water. Seeds were then blot dried and inoculated on half-strength MS (Murashige and Skoog 1962) medium with 1.5% sucrose, pH 5.7 – 5.8 and incubated with a photoperiod of 16h light (lux). From 8 day old seedlings hypocotyl were excised and placed on regeneration medium (BAP – 2.0 mg l-1 with TDZ – 1.0 mg l-1). and incubated for 2 days before transformation. |
• Agrobacterium transformation of AMP gene |
| Tomato hypocotyledons were co-cultivated with Agrobacterium strain LBA4404 (pCAMBIA2301Ace-AMP1) to develop transgenic plants. As a perquisite in developing transgenic plants, it’s essential to study the optimum cell density of Agrobacterium with duration and effective concentration of antibiotics on transformed plants. It is a prerequisite to study various co-cultivation parameters in Agrobacterium transformation. |
• Optical density and duration |
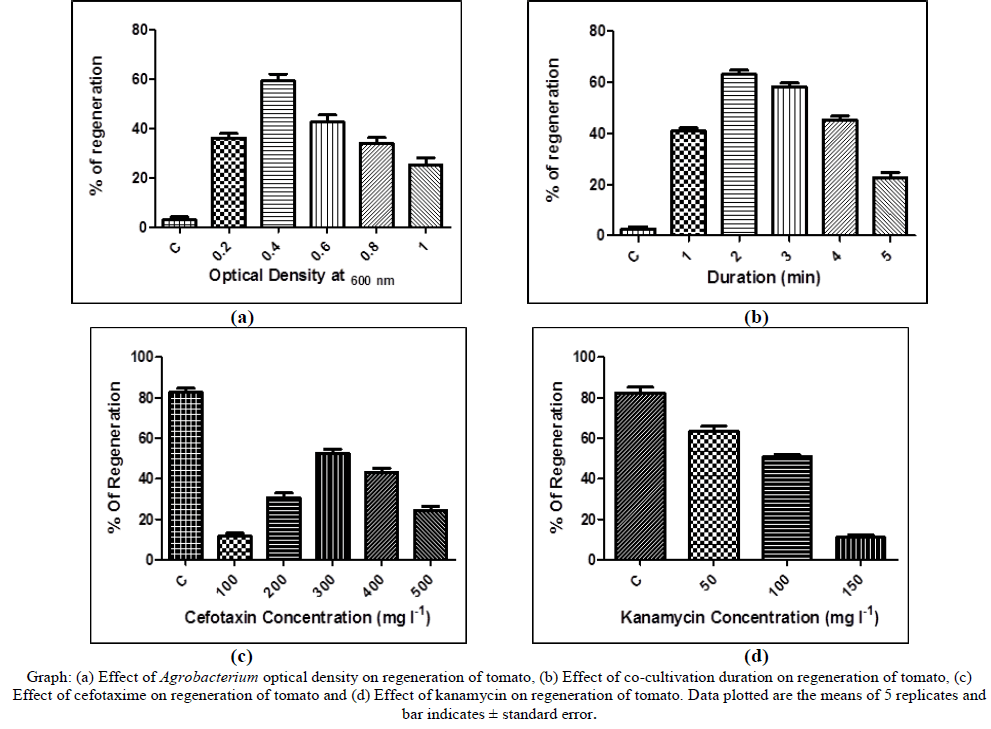
| The effect of optical density and duration of co-cultivation was studied. Agrobacterium culture with different O.D600 nm ranging from 0.2 – 1.0 and with varying duration of 1 – 5 min was tested for co-cultivation. Number of plants regenerated was recorded and the best OD and duration were used for further co-cultivation. |
• Antibiotic sensitivity test |
| Cefotaxime (100 – 500 mg l-1) was used to control the over growth of Agrobacterium and kanamycin (25 – 125 mg l-1) was used for selection of transformants. For this purpose hypocotyledons were co-cultivated and plated on MS media containing different concentration of cefotaxime. And hypocotyledons without co-cultivation were plated on media containing different concentration of kanamycin. Effects of both the antibiotics were tested and the best concentration was selected for co-cultivation and selection of transformation. |
• Co-cultivation of Hypocotyledons |
| Hypocotyledons were incubated with Agrobacterium culture (pCAMBIA2301Ace-AMP1) and stirred regularly. Immediately the culture was drained and washed thrice with MS medium to remove excess Agrobacterium. Hypocotyledons were semi air dried on a sterile tissue paper and transferred to petri dish containing regeneration medium and incubated in dark for 3 days at 26 °C. |
• Regeneration and selection of transformed Hypocotyledons |
| Hypocotyledons were then transferred to regeneration medium containing cefotaxime for three days. Further they were transferred to selection medium containing cefotaxime and kanamycin and sub-cultured every 15 days till regeneration was observed. Regenerated shoots of 1 – 2 cm in length were cut from the callus and transferred to elongation and rooting medium (1/2 MS containing IBA 0.5 mg l-1 and 0.02% charcoal). The regenerated shoots were maintained under selection medium with antibiotics throughout regeneration till rooting to avoid the growth of chimeras. |
• Hardening of transformed plants |
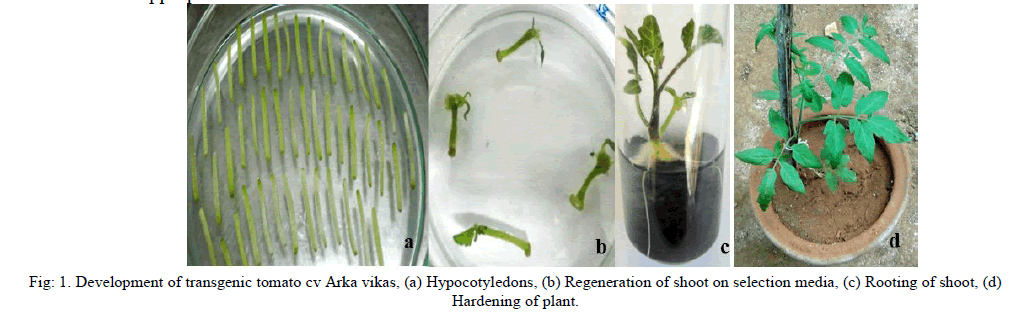
| After complete formation of root, plants were washed under running tap water to remove media contents followed by transfer to soilrite in pots. The pots were covered with poly ethylene bags to keep high level of humidity which was subsequently reduced by making holes in bags after 5-7 days until plants were acclimatized. |
• Control plants |
| Control plants were also regenerated through same way as transgenic shoots except that hypocotyledons were not treated with Agrobacterium and they were maintained on BT30 regeneration medium devoid of antibiotics and served as positive control. Hypocotyledons which were grown on selection media served as negative control, this was done to observe the effect of selection medium on non-transformed shoots. Callus formation and regeneration was observed in both the controls. |
• Molecular analysis of the transformants |
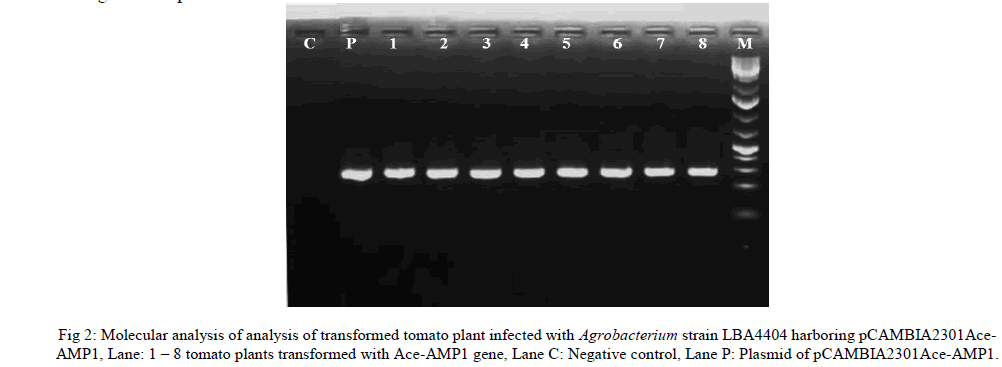
| To confirm the presence of Ace-AMP1 gene in putative transformed plants, total gDNA was isolated from both transformed and untransformed (negative control) plant samples and were used as templates for PCR. Plasmid DNA was also isolated and used as a positive control. PCR was performed using gene specific primers of Ace-AMP ‘Forward’ 5' – GCGCCATGGATGCAAATATGCAACATA - 3', and ‘Reverse’ 5' – ATACACGTGGTT AATCCTGCCGCATTG - 3' with standard protocol. The amplified products were electrophoresed in agarose gel. |
| The observations were made for percentage of shoot regeneration, number of shoots per explant, shoot elongation and rooting. All the data were subjected to analysis of variance (ANOVA). A post-hoc analysis test LSD (Least Significant Difference) was carried out to test the significance of different treatments means. |
III. RESULTS |
| Transformation and regeneration was optimised for tomato (cv. Arka vikas). Hypocotyledons were transformed by using Agrobacterium tumefaciens LBA4404 strain harbouring pCAMBIA2301Ace-AMP1 gene. It was found that, Agrobacterium density at 0.4 (O.D600 nm) gave the maximum number of transformed plants when cocultivated 2 min. At higher OD, the hypocotyledons showed overgrowth of Agrobacterium contamination, turned brown and died. Hypocotyledons were selected on cefotaxime 300 mg l-1 and kanamycin 100 mg l-1. Hypocotyledons did not regenerate on higher concentration of antibiotics. |
 |
| 90 % of callus formation was observed on the cut ends of hypocotyledons in 15 - 20 days. Adventitious buds were formed and 59 shoots emerged in 4 – 5 weeks on media containing selection antibiotics. All the regenerated shoots were cut at the base and transferred rooting medium. Roots were observed in 10 – 15 days and 22 shoots rooted. Plantlets which produced enough root were transferred to pots containing soilrite and 16 plants survived during hardening. Finally hardened plants were shifted to earthen pots and 13 plants survived at the end of hardening. |
| Controls play a crucial role in determining successful transformation. In positive control, callus formation was seen and this indicates that suitable regeneration system was followed. In negative control, callus was not able to form on hypocotyledons and necrosis was observed due to toxicity of kanamycin, this showed the concentration used in selection was appropriate. |
 |
Analysis of putative transformed plants in T0 |
| T0 plants were fertile and plants did not show any morphological difference when compared with control plants. Although putative transformed T0 were initially screened on selection media in vitro condition. It is essential to screen the plants further for the presence of gene by PCR analysis. PCR showed the amplification of 360 bp of Ace- AMP1 gene in 8 plants. |
 |
IV. DISCUSSION |
| Agrobacterium mediated transformation is most widely used approach in introducing gene of interest in plant genome. Even though several research has been done on Agrobacterium transformation, requires standardisation for a particular cultivar. Various factors are involved in developing highly efficient transformation, among those Agrobacterium infiltrations with different duration, concentration of cefotaxime and kanamycin is studied. |
| The effect of bacterial density and duration was studied it was found that 0.4 OD with 1 min of co-cultivation duration gave highest transformation efficiency. Increasing the bacterial cell density and duration negatively affected the explants. The explants turned black, soft and lost their regeneration capacity. In addition blotting of explants is important, any residue on explants resulted in death of the explants. Cefotaxime concentration was studied on inhibition of Agrobacterium overgrowth. Sharma et al 2009, selected the regenerated shoots in 500 mg l-1, in this study 300 mg l- 1 inhibited the growth of Agrobacterium and also did not affect regeneration process whereas higher concentration of cefotaxime affected the regeneration growth. Kanamycin 100 mg l-1 was best which gave highest transformation efficiency with low chimeras. |
| Hypocotyledon was used in transformation of tomato as it is the preferred explant type, with higher transformation efficiency when compared with other explants (Gubis et al 2003). Van Roekel, et al. (1993) reported there is a significant effect of media and Agrobacterium strains used on transformation frequency of tomato. Highfrequency of transformation also depends on the efficiency of plant in vitro regeneration and also on the elimination of bacterial cells from transformed tissues (Tang et al., 2004). In this experiment a 13% of transformation frequency was observed however frequencies have ranged from 6% in cv. Pusa Ruby (Sree-Vidya et al., 2000) to 40% in cv. Micro- Tom (Sun et al., 2006). |
| In this experiment, we have improved the transformation of tomato cultivar ‘Arka vikas’ using different parameters. |
References |
|