ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
S. Sreehari Sastry1,*, N.K.S.P.S.Sarma1, S. Salma Begum2 and Ha Sie Tiong3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The molecular interactions between the polar systems of p-hydroxy dodecyl benzoate(12HB) and Isoproponal for various volume fractions were studied at room temperature to determine the frequency dependent complex dielectric permittivity using Abbe’s refractometer for optical (1015Hz) frequency, the cavity perturbation technique for the microwave frequency at 10GHz (X-Band),and LCR meter at 1KHz. The Relaxation times and dipole moments for the binary mixtures were calculated using Higasi’s method and compared them with theoretical values. The formation of Hydrogen bond between the polar system as caused for the variations in the dipole moments and relaxation times in binary mixtures for different concentrations. The conformational analysis of the formation of hydrogen bond between dodecyl hydroxyl benzoate and isopropyl alcohol is supported by the experimental FTIR spectral studies and theoretical computational methods.
Keywords |
| Complex dielectric permittivity, dipole moment, relaxation time, Hydrogen bond. |
INTRODUCTION |
| Most of the dielectric relaxation processes reported in the literature were referred for understanding dilute solutions of polar substance in non-polar liquids. Though the non-polar liquid does not itself undergo relaxation but it alters the relaxation time of the solute molecule by reducing the internal field and changing the viscosity. The study of dielectric dispersion and absorption of a binary liquid mixture provides a very sensitive tool for detecting molecular interactions It has been observed that the formation of complexes or the presence of association leads to relaxation times considerably higher than those for the uncomplexed (unassociated) species. It has been shown by Schallamach [1] and other workers [2-4] that a binary liquid mixture, in which both components are either associated or non-associated shows a single relaxation time, whereas two distinct relaxation times are observed in the mixtures in which one component is associated and the other is non-associated. Dipole moments are determined for the polar solute system diluted in non-polar solvent benzene to minimize the dipole-dipole interaction. The dielectric behavior of some hydrogen bonded polar liquid mixtures was studied by Vishwam [5].Relatively little work has been done on mixtures of polar components. The dielectric constants and losses of binary mixtures of polar components as functions of temperature are found to have two maxima for associated and non-associated liquid but only one maximum for mixtures containing either associated liquids or non-associated liquids separately investigated by Schell mach [1] . However, measurements made at several microwave frequencies and at different temperatures have indicated that the dielectric behaviour of binary mixtures of non-associated pure liquids [6-10] have been reported in the literature. Such determinations have been made and reported in the literature on a wide range of substances both in solution and pure liquid state in the last several decades much attention in the recent years is focused in the studies for the mixture of associating compounds. Dielectric dispersion studies of polar liquids such as alcohols, benzoates and their binary mixtures where carried out several decades much attention in the recent years is focused in the studies for the mixture of associating compounds. Dielectric dispersion studies of polar liquids such as alcohols, benzoates and their binary mixtures where carried out in the present work. |
II. EXPERIMENTAL METHODS |
Materials: |
| The p-Hydroxy dodecyl benzoate (12HB) (99.5% purity) supplied by M/S Frinton laboratories Inc, USA, and Isopropanol and Benzene supplied from Qualigens, India. Isopropanol and benzene are ExcelaR grade and are double distilled before use. Benzene is used as solvent, where as the the binary system of Isopropanol and 12HB used as a solute in the preparation of solution. p-Hydroxy Dodecyl Benzoate (12HB) (99.5% purity) supplied by M/S Frinton laboratories Inc, USA, Isopropanol and Benzene supplied from Qualigens, India. Isopropanol and benzene are ExcelaR grade and were double distilled before use. Benzene is used as solvent, the binary system of Isopropanol and 12HB is used as the solute in the preparation of solution [11,12]. |
III. DETERMINATION OF PARAMETERS |
| The dielectric constants for all the prepared solutions are measured using Abbe refractometer (in optical frequency). and microwave cavity bench (in X-band),and LCR meter(around 1KHz). |
A. Abbe Refractometer |
| The refractive index measured through this device will give dielectri constant. Using the relation |
B. Microwave Cavity Technique: |
| The basic setup of Microwave cavity bench is shown below. In this technique less quantity of sample is used (5ml) when compared to plunger technique. |
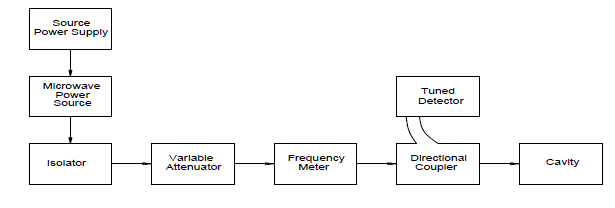 ' ' |
| Capacitance 0 C , C1 and C2 are corresponding to empty cavity, cavity with empty holder and cavity containing the holder with sample within it respectively. Resonant frequencies 0 f , 1 f and 2 f are also correspond to empty cavity, cavity with empty holder alone and lastly cavity containing holder with the sample within it respectively. Vc and Vs are volumes of the cavity and volumes of sample respectively.Q0, Q1 and Q2 represent quality factors of empty cavity, empty sample holder and cavity containing sample with holder, respectively. |
C. LCR meter: |
| The low frequency static dielectric constant (1 kHz) is measured by using Digital LCR meter (PLCR-8C). Dielectric constant for unknown liquids can be determined by measuring the capacitance of the empty cell in air (Cair) and the capacitance of the same cell in liquid under study (Cliquid). If the parasitic capacitance for the cell is known, the relation gives the static permittivity of the liquid sample. |
| The weights and corresponding volume fraction of 12HB in Isopropanol used in the present study is shown in table 1. |
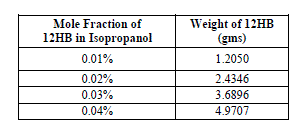 |
| All the weights are measured using a digital electronic balance Mettler Toledo make, model AB135-S/Fact to the accuracy of 0.00001 mg. |
IV. RESULTS AND DISCUSSIONS |
Results : |
| The different concentrations of solute system (12HB + Isopropanol) are dissolved in non-polar solvent benzene at various volume concentrations [14,15].The dielectric constant for each concentration is measured at optical frequency at low frequency 1 kHz and at microwave frequency 10 GHz at room temperature. The dielectric data obtained from these measurements using LCR bridge (static dielectric constant) using the microwave bench adopting cavity technique (in X-band) at microwave frequencies, Abbe refractometer for measurement of refractive index is given in Table II. |
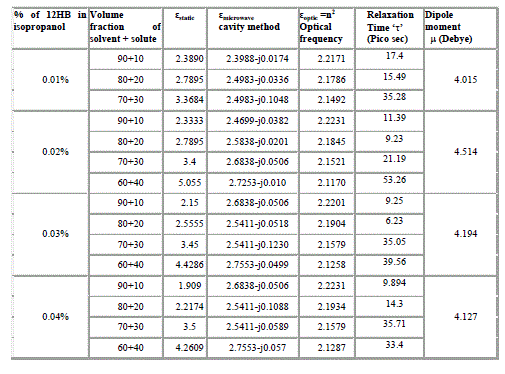 |
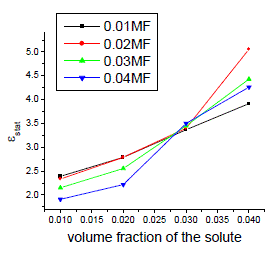
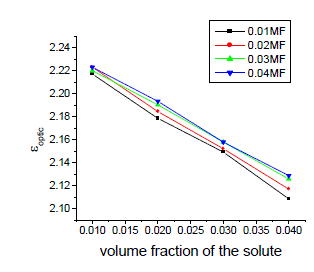
| The variation of static, optic and microwave dielectric data with different volume fractions of solute for various mole fractions are shown in figures 2 to 5 |
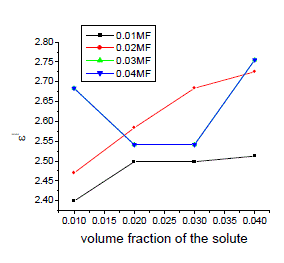 |
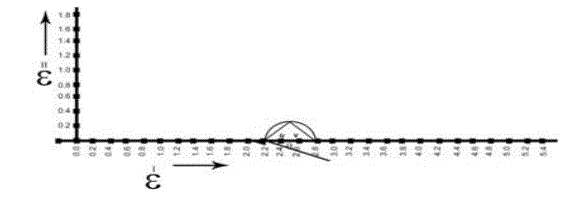
| In order to calculate relaxation times by using cole-cole plots[13], the data obtained from all the above said methods are used The typical cole-cole arc drawn is as shown in the below fig.6 |
 |
| The dipole moments are calculated from Higasi method |
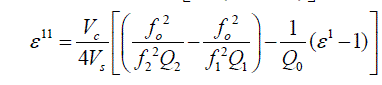 |
| where M2 is molecular weight of solute, d1 density of solvent, ïÃÂÃÂ¥1 the dielectric constant of solvent (benzene), a0 and aïÃâÃÂ¥ the slopes of ïÃÂÃÂ¥o and ïÃÂÃ¥ïÃâÃÂ¥ are respectively with respect to the concentration. The other constants have the usual meaning. |
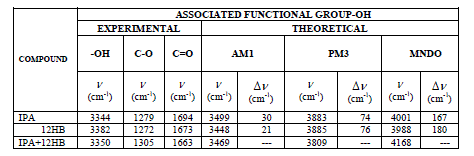
| FTIR: In the present study the theoretical values of FTIR is studied by using “PC SPARTAN PRO”. With the Spartan the structures of IPA, 12HB, and IPA+12HB are produced as shown in the figure (1). The table III gives the FTIR (theoretical) wave number values for pure and solute mixture of IPA and 5HB in three different modes (AM1, PM3, MNDO) .The Shifts between the pure and Solute mixture in the associated functional group –OH is also studied. |
 |
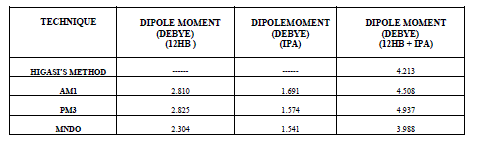
| The experimental and theoretical dipole moment values for individual and mixture systems are given in the table IV. |
 |
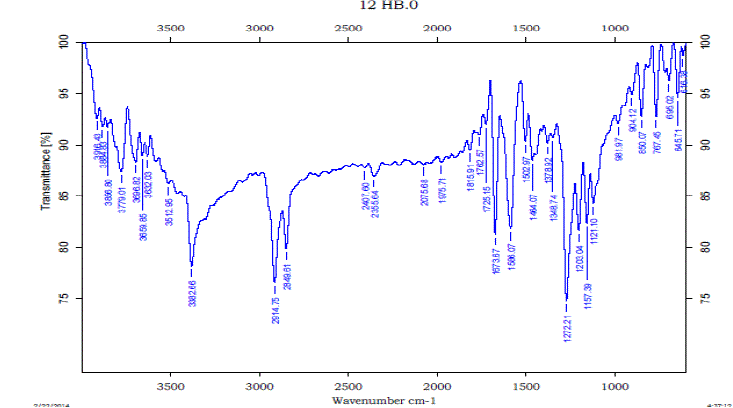
| The Formation of Hydrogen bond is confirmed by experimental FTIR also. The vibrational modes of transmission of IR signals for IPA, 12HB and 12HB+IPA are shown in figures (7) to (9). From the experimental FT-IR spectra is observed that for the equi molar mixture of 12HB and IPA there is a hypsochromic shift of 6 cm-1 wave number in the position of –OH, these shifts are caused by the strong interaction between the compounds Thus the IR analysis convinces intermolecular hydrogen bonding . |
 |
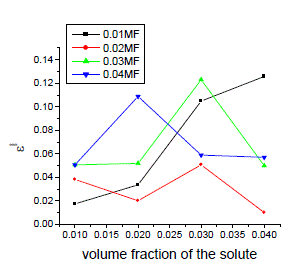 |
| It is evident from the data given in the Table II, the dielectric constant decreases with increase of frequency. The microwave data show that these compounds are polar in nature and correspondingly the dielectric constant is a complex quantity. The relaxation time is found to increase in magnitude at a given concentration of 12HB in Isopropanol for the increase of dilution in Benzene indicates that the molecules are free to rotate as benzene concentration increases. The same tendency is also seen for different concentrations of 12HB in Isopropanol. The dipole moment values are decreased with increase of 12HB in Isopropanol which is probably due to the formation of hydrogen bonding in the system [16-18]. This can be concluded from ab-initio calculation which gives molecular conformations. However from the present study, the dipole moment is varied between 4.36 and 4.53 for various concentrations of 12HB in Isopropanol. The relaxation times are found to increase in magnitude for a given mole fraction of alkyl hydroxyl benzoates in isopropyl alcohol for the increase of solute volume fraction in benzene .In the microwave region also the dielectric constant increases with the increase of concentration of 12HB in binary system. But at optical frequency region the dielectric constant decreases when the concentration of solute increases in the binary system. It is due to dipolar polarization contributing decrease at high frequency. At high frequency the dipolar polarization disappears and only atomic polarization or electronic polarization is present [19-22]. The relaxation time increases with the increase of concentration of solute This behaviour may be attributed to the formation of hydrogen bond between isopropyl alcohol and 12HB. The theoretical and experimental FTIR are correlated with each other and AM1 model is suggested for confirmed the presence of hydrogen bond between the polar systems mixture. |
 |
 |
V. CONCLUSION |
| The dielectric properties like relaxation times, dipole moments for various concentrations at room temperature of p-hydroxy dodecyl benzoate (12HB) with Isopropanol in benzene studies indicated the formation of hydrogen bond in the mixtures. There is increase in the dipole moment as the concentration of benzoate is increased in solute system in non-polar solvent benzene and it is due to the formation of hydrogen bond between the solute systems. The confirmation of hydrogen bond is confirmed through theoretical studies. Further experimental FTIR studies also revealed thformation of hydrogen bond between oxygen of Insopropanol and hydrogen of benzoate. . |
ACKNOWLEDGMENTS |
| The authors gratefully acknowledge UGC DRS LEVEL III program No.F.530/1/DRS/2009 (SAP-I), dated 09-02-2009 and DST FIST program No DST/FST/ PSI – 002/2011dated 20-12-2011, New Delhi, to the department of Physics, ANU for providing financial assistance. |
References |
|