ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Nitin Motiram Jadhav1, Vinod Nayak 2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The battery electrolyte which contain heavy metals such as lead, zinc are neutralized by adding sodium hydroxide and depending upon cumulative concentration of lead and zinc and other heavy metals ; sulphide ions are introduced equal to cumulative concentration of all lead and zinc and other heavy metals. The lead, zinc and other heavy metals form metal sulphide which are generally very insoluble and gets precipitated which can be very easily removed by settling them at bottom and followed by decantation process.
Keywords |
| Waste battery Electrolyte, Heavy metal, Neutralization [1], Precipitation [2], Decantation [3], Pollution control, Waste management. |
I. INTRODUCTION |
| In many industries lead acid battery is used for uninterrupted power supply sources. These batteries contain sulphuric acid as electrolyte. Due to operational uses, periodically battery electrolytes are being removed from batteries. These waste battery electrolyte contain heavy metals such as lead, zinc, chromium etc. These heavy metals can cause serious environmental hazards due to their high bio specificity, toxicity and mobility. These heavy metals need to be removed before disposal. Also the pH of electrolyte is <1.0 and need to be neutralized before disposal. These heavy metals can be removed by charcoal adsorption method but this method produces large quantity of post disposal poisonous charcoal waste, which is difficult to handle. |
| The chemical precipitation technique was studied on laboratory basis and its practical applicability on large industrial scale was applied which show very good results. And it is concluded that this is the best method technique due to high removal capacity, low cost, easy availability, less operation difficulties and there is no post disposal wastage generation as observed in charcoal adsorption method [4]. |
II PROCEDURE EXTRACT |
| The heavy metals content present in the wastewater can be removed effectively to acceptable levels by introducing ions such as hydroxide, sulphide, sulphate and carbonate etc. which convert soluble heavy metal ion in to insoluble form and get precipitated at the bottom. As per literature, solubility of metal sulphide is very low compare to hydroxide and which cause lower residual metal concentration in treated waste water. Therefore sulphide ion salt (sodium sulphide) is decided to use for heavy metal precipitation process in battery electrolyte |
 |
1. pH of the electrolyte |
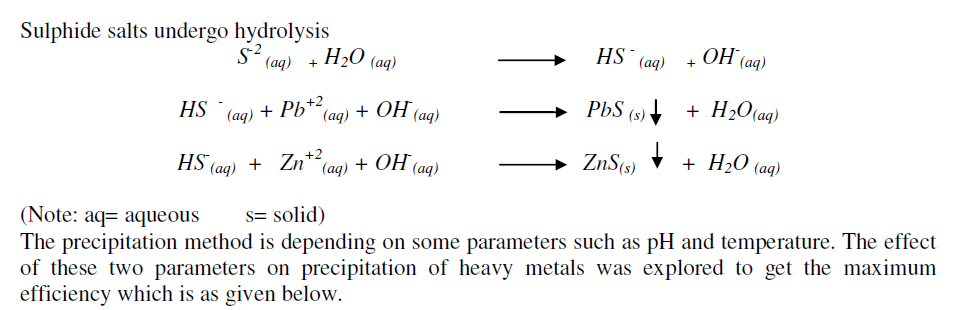
| pH of the electrolyte solution plays a important role in the precipitation of heavy metals. Solubility of heavy metals ion in low pH range is higher due to which, precipitate are not formed and also added sulphide ions react with hydrogen ions to form hydrogen sulphide gas which get evolved [5]. Hydrogen sulphide gas is toxic and has foul smell. Hence sulphide precipitation was carried out in alkaline condition and pH was maintained in the range of 7.-8.0. |
 |
| Due to which more amount of sulphide ions are required for precipitation. Hydrogen sulphide gas is very poisonous and has got foul smell. Hence sulphide precipitation has to be carried out in just alkaline condition to promote sulphide ion formation. |
2. Temperature of the electrolyte |
| The temperature of the electrolyte increases sharply during neutralization. Hence cooling facility has to be provided. The addition of sodium sulphide has to be done in room temperature because due to high temperature sulphide ions get evolved forming hydrogen sulphide gas which will reduce sulphide ions concentration required for the precipitation. Hence the sodium sulphide salt should be added at room temperature for greater efficiency [6]. |
III STANDARDIZATION OF DISPOSAL METHOD |
| To standardize this method’s practicality and its implementation, the pilot experiment was carried out in laboratory with successfully manners. From the lab experience, the procedure was prepared for disposal of large quantity of waste electrolyte, keeping the view of industrial applicability. The details of laboratory scale (small scale) and filed scale (large scale) procedure are as explain below. |
A) LABORATORY EXPERIMENT:- |
| To see the performance of neutralization and precipitation for removal of heavy metal from electrolyte, it was decided to do experiment on laboratory Scale first. |
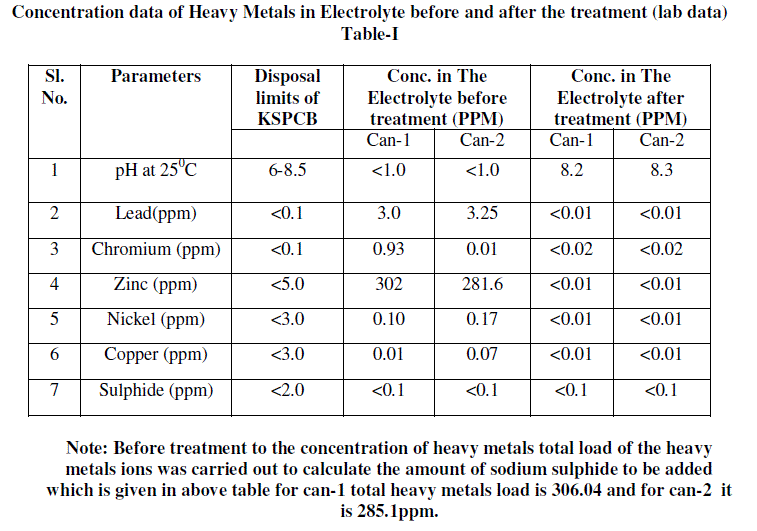
| 1. 100 ml battery electrolyte sample was taken and its initial concentration of heavy metals, pH other parameters were analysed. Analysis report is included in table-I as a Conc. in The Electrolyte before treatment. |
| 2. Sample was neutralized using 48% sodium hydroxide to make pH 7-8. During neutralization electrolyte beaker was cooled from outer side to avoid heating up. About 29 ml of sodium hydroxide was added for neutralization. |
| 3. Calculated amount of Sodium sulphide (Equivalent to heavy metals presents) was added to precipitate heavy metals present in electrolyte. During this process stirring was carry out for smooth precipitation. For precipitation total 0.14365(for can-1: 0.07437 gm, can-2: 0.06928gm) grams of sodium sulphide was added. |
Amount sodium sulphide to be added: |
| Concentration of lead in ppm +concentration of Zinc in ppm+ concentration of other heavy metals etc = A |
| Sodium Sulphide to be added in gm = cumulative concentration (A) in ppm X Inventory in tone X 2.433 |
| (2.433 is factor for sulphide ion for sodium sulphide salt.) |
| 4. After this electrolyte was kept stagnant for 2-3 hrs for complete settle down the heavy metal precipitate at the bottom of carboy. |
| 5. The solution was separated from precipitate by decantation and decanted solution was analysed for the different parameters which show that heavy metals concentration were below detectable limits. Analysis report is included in table-I as Conc. in The Electrolyte after treatment. |
| 6. Decanted solution was analysed for sulphide and found between <0.1ppm. |
| 7. It was observed that by giving washing to precipitate, precipitate weight decreases due to removal of sodium sulphate salt which is present along with the precipitate. Hence 2-3 times washing was given by water. |
| 8. Precipitate was dried in sun light and oven. |
| 9. Approximate 0.2 gram precipitate was removed in 100ml of acid electrolyte solution. |
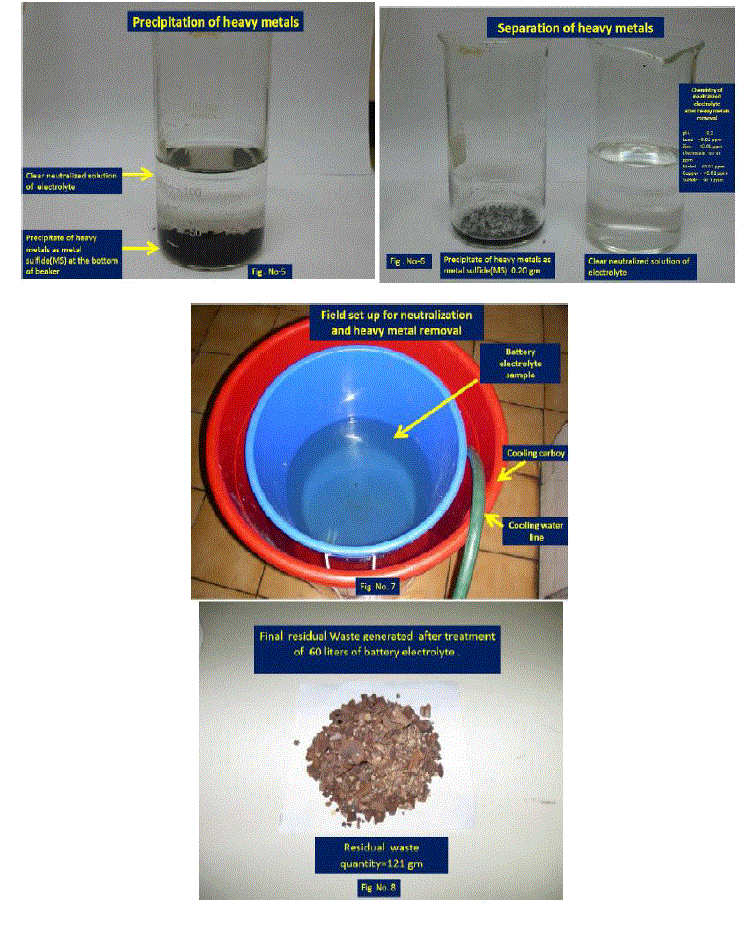
| 10. Photo presentation of neutralization, precipitation and removal procedure of heavy metals are given in annexure no.1. |
B) Analysis data of Electrolyte |
 |
IV FIELD EXPERIMENT |
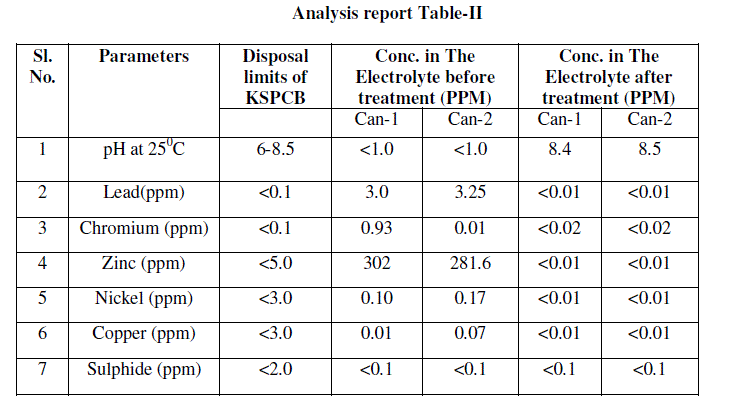
| After successful experiment on laboratory scale, it was decided to study the efficiency of this method and to standardize the procedure in field. Around 60 litres battery electrolyte was taken for treatment in on pilot study from CAN- 1and CAN-2. Its initial concentration of heavy metals, pH other parameters were analysed; analysis report is included in table-II as Conc. in The Electrolyte before treatment. |
| ïÃÆÃË 60 litres battery acid was taken in 60 litres PVC drums. This drum was kept in 120 litres drum. Domestic water was used for cooling with flow of 5 LPM during neutralization of acid. DM plant alkali was slowly added for neutralization. Temperature of cooling water was maintain regularly and it was ensured that acid temperature remain below 45-500C. For neutralization of 60liter acid around 22liter of alkali was required. |
| ïÃÆÃË Quantity of sodium sulphide was calculated and 29.3216 Gms of sodium sulphide were added. During addition of sulphide solution was stirred. Colour changes to black. After one hours of stirring solution was left for 24hrs for settle. After settlement of precipitate top water was found clear. |
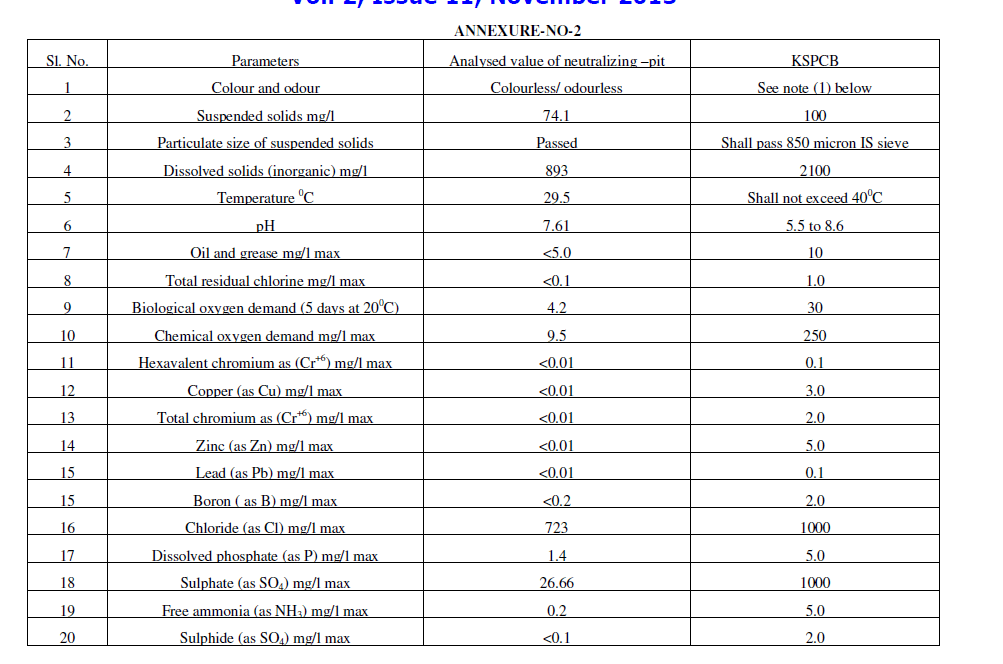
| ïÃÆÃË Water portion was analysed for chemical parameters. Analysis report was found within pollution control limits. This analysis report is included in table-II as Conc. in the Electrolyte after treatment. After ensuring heavy metals and pH within limits. This was first dispose in neutralizing pit for further dilution and disposal. Before disposal of waste from neutralizing pit it was analyse for details chemical parameter and found acceptable as given in annexure No.1 as per Karnataka pollution control board [7]. |
| ïÃÆÃË Sludge obtained which contains heavy metal was wash with water to remove soluble sodium sulphate salt [8]. Precipitate was first dried in sun light and hot air oven. Around 142 Gms of precipitate [9] was obtained. Photo of Precipitate is included in figure- no 8. This is finally kept in bottle for disposal in waste management plant. |
 |
V SAFETY PRECAUTION |
| 1) During handling of acids and alkalise protective equipments such as Acid/ alkali résistant gloves, aprons, goggles etc.has to be use. |
| 2) During neutralization, care was taken to avoid spillage of acid and alkali. |
| 3) Neutralization was done slowly to avoid excessive heat generation. |
| 4) Separate the solution from precipitate and analyse the decanted solution for heavy metals. If the results are still exceeding pollution control limits repeat procedure. |
 |
 |
CONCLUSION |
| Recommendation: |
| A. Using this method we can also able to remove heavy metals from industrial drainage water waste. |
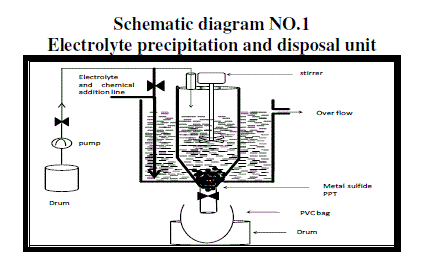
| B. Disposal of waste battery electrolyte has to be carried out very safely hence one set up was designed to handle large quantity of acid waste for industrial large waste. The design is as shown in schematic diagram No1. |
 |
 |
References |
|