ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Rahma Belcadi-Haloui1, Abderrahmane Zekhnini2, Miloud El Hadek3 and Abdelhakim Hatimi1
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The effect of light and oxygen on the traditional argan oil stability was studied for a period of 12 months. The samples were stored in transparent glass vials at room temperature in the presence of air, nitrogen or in the darkness. The autoxidation was evaluated by monitoring four parameters: tocopherols, unsaturated fatty acids (oleic and linoleic acids), conjugated dienes and peroxide value. The results obtained showed that the oil deteriorated more rapidly in the presence of light compared to the dark. This degradation was more pronounced in the presence of air compared to the nitrogen. Indeed, the results indicated that the time before the maximum level of hydroperoxides, resulting from the degradation of unsaturated fatty acids, was to about 200 days. This period corresponded to the oxidative stability of the oil that was highly depending on the presence of the antioxidants such as tocopherols. Thus, the loss of these molecules led to accelerating unsaturated fatty acids degradation and producing conjugated dienes and hydroperoxides which are the primary products of oxidation. Since the α-tocopherol was destroyed during the first days of storage, these are the γ- and δ-tocopherols which expressed most significant and longer antioxidant effect. The use of nitrogen as storage environment permitted slightly delaying tocopherols deterioration and therefore unsaturated fatty acids oxidation.
Keywords |
| Argan Oil, Autoxidation, Fatty Acid, Light, Nitrogen, Oxygen. |
INTRODUCTION |
| Argan oil used in food is extracted in a traditional way and is known to deteriorate rapidly under usual storage conditions [1, 2, 3]. The autoxidation would be induced by several factors. Indeed, the extraction occurs after roasting almonds in order to highlight the organoleptic properties [4]. It is known that high temperatures destroy tocopherols which are powerfull antioxidants and therefore a good part of their content could deteriorate during the traditional extraction [5]. Furthemore, the use of water during the manual extraction could initiate the oxidation of unsaturated fatty acids [2]. Even so, the oxidation of the oils depends on the storage conditions such as temperature, lighting and oxygen [6, 7]. The rapid deterioration of traditional argan oil would be a limiting factor for marketing and consumption in other parts of Morocco, and even in other countries. However, this instability remains little studied. Few works were undertaken to monitor the degree of oxidation according to the mode of extraction or after heating the oil [1, 8, 9]. The aim of this work is to study the stability of traditional argan oil stored under different conditions to evaluate the effect of ambient temperature, oxygen and light. For this purpose, we followed the content of major unsaturated fatty acids (oleic and linoleic acids), tocopherols, conjugated dienes and peroxide value, during a period of 12 mouths. |
MATERIALS AND METHODS |
1. Oil used |
| All manipulations were carried out on a single sample of argan oil which was traditionally extracted under our control. Oval fruits of argan tree (Argania spinosa, Skeel) from Admine region were used. The seeds were crushed manually and obtained almonds were roasted over low heat and then ground using a traditional stone mill. The oil extraction was performed by mixing the obtained slurry and on which drops of warm water were added from time to time. The oil rose to the surface and was collected by decantation. It was immediately analyzed to determine the initial composition. |
2. Storage conditions |
| The oil was distributed into 12 clear transparent glass vials of 40 ml at a rate of 30 g per vial: 6 vials in the presence of air and 6 in the presence of nitrogen with which air was removed. For these 2 lots, the bottles are tightly closed and stored under the following conditions: |
| - 3 bottles in the presence of day light at room temperature (25 ° C). |
| - 3 bottles in obscurity at room temperature (25 ° C). |
3. Fatty acid composition |
| After its extraction, argan oil was methylated with Boron trifluoride (BF3). Three extractions with hexane were then made to obtain fatty acid methylic esters. These esters were evaporated under nitrogen conditions and 50 μg were directly injected into a Cpcil-88 column (length 50 m; diameter 0.25 mm) of a Carlo Erba gas chromatograph whose temperature increased from 150 to 225 °C (5 °C/min). After their separation in the column, fatty acids were detected using a flame ionization detector at 250 °C. The identification of fatty acids was made by comparing their equivalent chain length with a standard mixture of fatty acids. The pressure of vector gas (H2) was 1 bar. |
4. Tocopherols content |
| Tocopherols composition of argan oil was performed with the HPLC method. The chromatographer comprised a Jasco 880-PU pump, a Jasco 875-UV spectrophotometer detector and a Varian 4400 integrator. Argan oil was diluted with 100% methanol and injected into a C18-grafted silica column (length 25 cm; diameter 3 μm). The tocopherol content was determined in comparison to a standard mixture. |
5. Conjugated Dienes (CD) |
| The oil was diluted with cyclohexane to a concentration of 1%. The CD content was assessed by absorbance at 233 nm using a Varian DMS 80 spectrophotometer. |
6. Peroxide Value [10] |
| To a mixture of 2 g of oil and 10 ml of chloroform were added 15 ml of acetic acid and 1 ml of potassium iodide. After stirring, the mixture was placed in the dark for 5 min. Then, were added 75 ml of distilled water and a small amount of starch powder as a color indicator. The titration was performed by the addition of sodium thiosulfate. |
RESULTS AND DISCUSSION |
| The initial composition of the used argan oil is shown in Table 1. Values obtained, either for tocopherols (394 mg/Kg of oil), unsaturated fatty acids (82 %), peroxide value (2.6 meq/Kg), were in accordance with the Moroccan Standard [11]. |
| However, the tocopherols content was lower than that obtained with the extraction using organic solvants [12]. This result gave evidence that the traditional extraction initiated the autoxidation of the oil. Indeed, argan fruit seeds are manual crushed and obtained almonds undergo a traditional roasting on low heat allowing highlighting the organoleptic properties of the oil. This Last is characterized by a nutty flavor appreciated by the consumer as mentioned by other authors [4]. |
| The evolution of the parameters studied showed that the oil samples placed in the presence of light were the first to undergo oxidative damage, unlike those stored in the obscurity (Figure 1-4). Tocopherols deteriorated first with rapid decrease of α-tocopherol (figure 1). The degradation was greater in samples stored in the presence of light and air. The total loss was noted after only 15 days of storage. The use of nitrogen allowed extending this period to 90 days. The degradation of the γ- and δ- tocopherols was slower. Their total disappearance occurred at days 250 and 264 respectively in the samples exposed to light and air, and those exposed to light and nitrogen. However, the three forms of tocopherols were maintained at their initial level when the oil was stored in the darkness. |
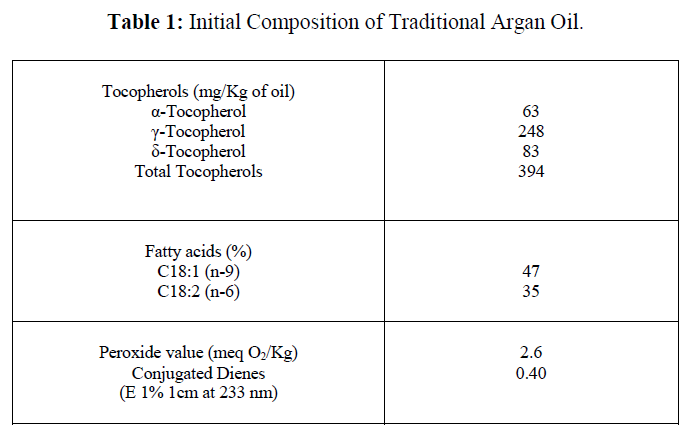 |
| The degradation of unsaturated fatty acids started when tocopherols were completely destroyed (figure 2). Linoleic acid was oxidized faster than oleic acid. Its residual content was not more than 5% at days 271 and 291 respectively in the samples exposed to light and air and those exposed to light and nitrogen. The oxidation of oleic acid showed two phases: an initial phase where the degradation rate was relatively low, and a second in which the deterioration became faster (from the day 257). However, the contents of both fatty acids remained unchanged in the samples stored in the darkness. |
| The fatty acids destruction resulted in an abrupt increase in hydroperoxides content which was estimated by measuring peroxide value (figure 3).The peroxides formation kinetics showed an initial phase where values increased slightly. This phase corresponds to 248 days for the oil stored in light and oxygen, and 270 days to that stored in the presence of light and nitrogen. The second phase was characterized by a rapid increase in the peroxide value and maximum levels were reached at days 274 and 298 respectively in the samples placed in the presence of light and air, and those stored in the presence of light and nitrogen. However, the maximum value recorded in the presence of nitrogen (6720 meqO2/Kg ) was significantly higher than that obtained in the presence of air (2687 meqO2/Kg). This increase was not observed when the oil was placed in darkness, and PV remained similar to that of the initial state. |
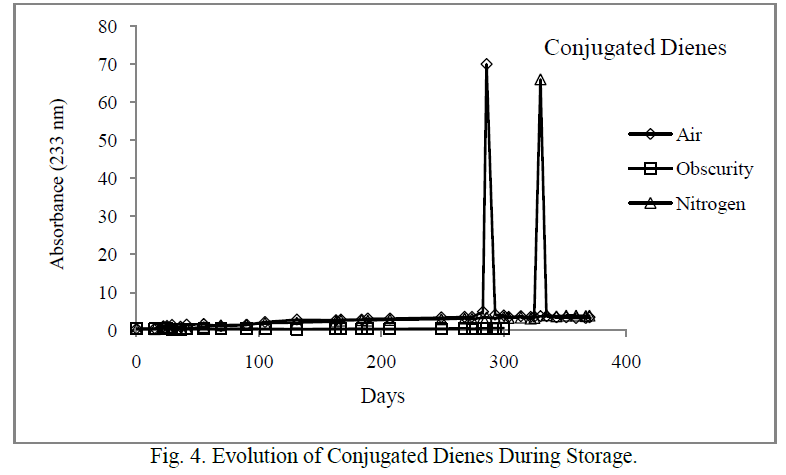
| The CD had similar evolution with PV (Figure 4). Their content increased sharply after 283 and 330 days respectively in the samples stored in the presence of light and air, and where the air was replaced by nitrogen. As peroxides, CD did not show any significant increase in the samples stored in the darkness. |
| Our results showed that the composition of unsaturated fatty acids would be maintained for approximately 200 days in the presence of light and air. During this period, the unsaturated fatty acids were protected from oxidation by the presence of antioxidants such as tocopherols. Indeed, the fatty acid degradation was accelerated as soon as the content of tocopherols was weakened. The tocopherols are a prime target for radical attack in vegetable oils where they occur naturally [13, 14, 15]. In our case, α-tocopherol manifested its protective effect early unlike the other two forms of tocopherols whose antioxidant action was much longer. In this regard, other authors reported an increasing antioxidant effect of γ-tocopherol in time, in olive and flax oils [3, 16, 17, 18]. |
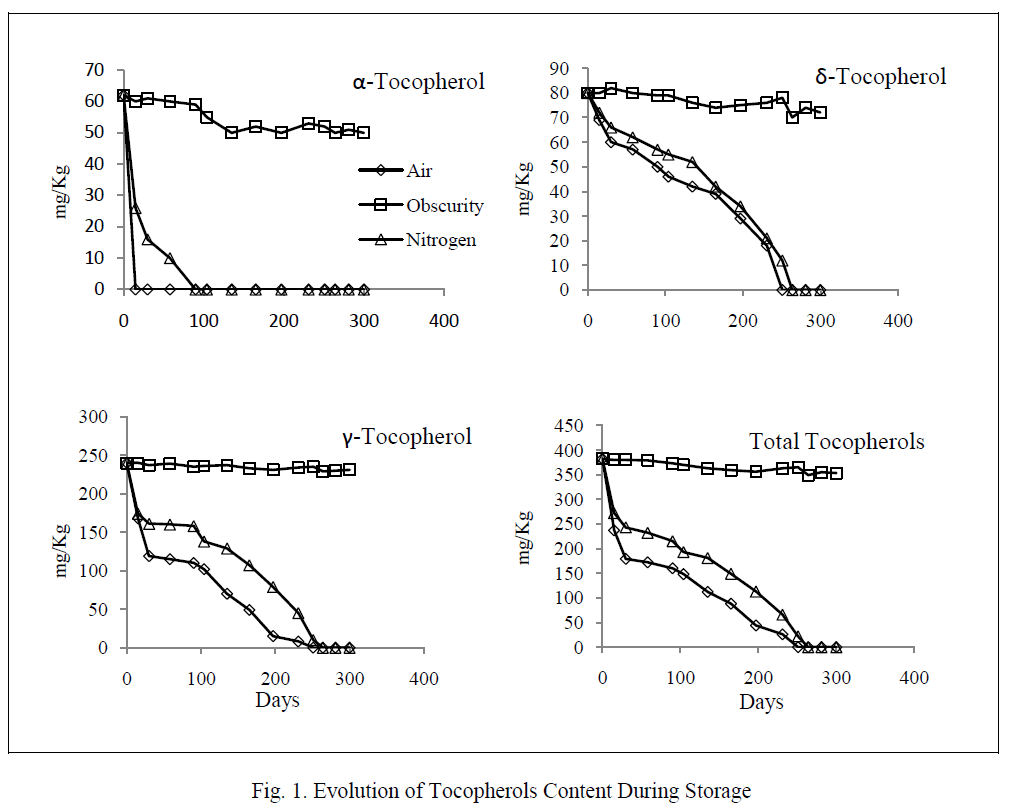 |
| As for variations of fatty acids, our study showed that the linoleic acid was oxidized faster than oleic acid in the light at room temperature. The same result was reported by Chimi et al. [19] on the autoxidation of decolorized argan oil stored in the dark at 35° C. This result is explained by the fact that linoleic acid is more unsaturated and double bonds are more vulnerable to free radicals attack [20]. |
| From these results, it was apparent that the storage of traditional oil in the presence of light caused degradation of unsaturated fatty acids and tocopherols. Many studies showed the adverse effect of light on the conservation of plant oils [9, 21]. Indeed, light is a factor which catalyzes the oxidation by bringing necessary energy to tear out a hydrogen atom from unsaturated fatty acid molecule (RH) which transforms in alkoxyl free radicals (ROO.) in the presence of oxygen. The alkoxyl radicals are unstable and transform into hydroperoxides (ROOH) by tearing out a hydrogen atom from another molecule of unsaturated fatty acids for generalizing oxidation reactions to all unsaturated fatty acids of the oil [22]. |
| Our results showed that the formation and decomposition of hydroperoxides, under the effect of light, varied depending on the presence or absence of oxygen (Figure 3). In fact, in the presence of both light and air, hydroperoxides decomposed into secondary oxidation products while they are formed. The recorded maximum value (2687 meqO2/kg) was lower than that noted in the samples stored under nitrogen where the rate of decomposition of hydroperoxides was lower, resulting in their gradual accumulation over time and leading to a higher maximum level (6720 meqO2/Kg). |
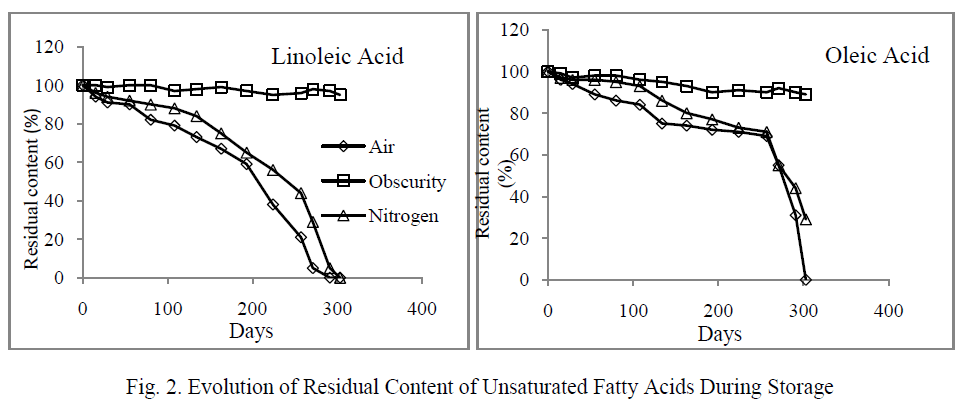 |
| Therefore, under the combined effect of light and air, the oxidation reaches the second phase. Conversely, in the absence of air (presence of nitrogen), the effect of light was particularly evident by the formation of primary products (hydroperoxides). Similar works performed on the olive oil showed that the simultaneous presence of light and air affected the evolution of the oxidation during storage. Oils stored in the presence of light and air contained secondary products, while those stored in the absence of light mainly contained primary products of oxidation [23, 24]. |
 |
| Also, some previous studies reported that the rate of formation of the secondary products from hydroperoxides varied depending on the initial composition of the oil. In olive and rapeseed oils, these products were generated immediately after the formation of hydroperoxides, whereas in sunflower and safflower oils, secondary oxidation products were formed when the amount of hydroperoxides was higher [25]. The hydroperoxides initially formed are odorless and tasteless [26, 27]. |
| The secondary oxidation products generated in the second phase from the decomposition of hydroperoxides contain volatile aldehydes (hexanal) and ketones which are responsible for unpleasant odor and flavor [28]. Other compounds could be toxic and harmful to human health [25, 29]. |
 |
| The use of nitrogen as storage environment had a limited impact against argan oil oxidation in our experiment. In the literature, the increase in the oxygen content led to accelerating the oxidation and the formation of secondary products [5, 30, 31] while the use of nitrogen as conditioning gas reduced the oxidation reaction [32, 33]. Our result (limited impact of nitrogen) could be explained by the amount of oxygen dissolved in the oil, allowing alkoxyl radicals production [34], and the roasting of almonds during the extraction, which may initiate the oxidation of the oil [2]. |
CONCLUSION |
| Our results allowed observing that the shelf life of traditional argan oil stored in the light, in the presence of air and at ambient temperature, was approximately 200 days. Under these conditions, α-tocopherol disappeared from the earliest days of storage. The γ- and δ- tocopherols disappeared much later and allowed a longer protection of the oil against oxidation. However, if storage in the absence of oxygen allowed only a slight improvement in the conservation of traditional argan oil, the use of darkness as a storage environment would give it long duration stability. In addition, the use of nitrogen as a conditioning gas would limit the production of secondary oxidation compounds and allow the oil to preserve organoleptic properties for a longer duration. |
| Finally, further investigations are needed to determine the effect of other intrinsic or extrinsic factors that may accelerate or slow down the oil oxidation. This concerns the content of the oil in other antioxidants like polyphenols and phospholipids, and the nature of the storage containers. |
References |
| Hilali, M., Charrouf, Z., El Aziz Soulhi, A., Hachimi, L., and Guillaume, D., “Influence of Origin and Extraction Method on Argan Oil Physico- Chemical Characteristics and Composition”, Journal of Agricultural and Food Chemistry, Vol. 53, pp. 2081-2087, 2005.
|