e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
1Department of Chemistry, Faculty of Science, University of Abuja, Abuja, Nigeria.
2Department of Biochemistry, Faculty of Science, Ahmadu Bello University, Zaria, Nigeria.
3Department of Biochemistry, Faculty of Science, Kogi State University, Ayingba, Nigeria.
4Department of Biochemistry, Faculty of Science, Kaduna State University, Kaduna, Nigeria.
Received: 19/02/2013 Accepted: 01/03/2013 Revised: 25/02/2013
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
The effect of Neem (Azadirachta indica) extracts (leaf and stem bark) on the sialic acid profile of Plasmodium berghei experimentally infected albino mice free from infectiionwas investigated in this study. Infected red blood cells from Plasmodium berghei-infected albino mice contains between 2 and 4 times as much sialic acid as uninfected red blood cell. The serum (free) sialic acid value was 1.45 mg/ml in the infected RBC and 0.87 mg/ml in the uninfected RBC. In all the experimental groups, the serum sialic acid and RBC-SA varied from one treatment to the other (1.13-1.40 and3.25-4.70 mg/ml) respectively. There was a significant difference (p≤0.05) between the values recorded for the treated samples when compared to the values of infected non treated mice (1.42-2.13 and 3.05- 3.71 mg/ ml) respectively. The RBC-SA values in the treated mice were significantly lower than the values recorded for the normal (non-infected) mice (4.27 and 5.21 mg/ml) respectively, but higher than the infected non treated mice. There was an increase in the serum sialic acid of the treated mice when compared to the values of normal mice which was also observed in the brain sialic acid profiles in the treated mice. The values recorded in the brain sialic acids profiles were higher than those obtained from the serum. The serum and brain free sialic acid content evaluated in survivors(treated mice) were almost normalized to the values obtained for the uninfected mice. Treatment with the neem extracts resulted in about 30 % reduction in free sialic acid release during treatments. The increase in the free sialic acid levels during infection, treatment and the subsequent decrease post-treatment suggested that the neem leaf and stem bark extracts possesses therapeutic efficacy and confers an advantage in ameliorating P. berghei infection which should be exploited in view of the global resistance of malaria parasites to mainstay anti-malarials.
Neem extracts, P. berghei, sialic acid, Albino mice.
Malaria is the most important of the parasitic disease of humans [1]. The majority of malaria deaths are due to cerebral complications [2]. Most of the deaths occur in Africa especially children under the age of 5 years [3-5]. Chloroquine has been the frontline anti-malaria drug of choice, but the resistance to chloroquine is now widely distributed in all geographical areas where P. falciparum is endemic [6,7]. The resistance of the malarial parasites to chloroquine and other drugs prompts the search for chemotherapeutic agents with novel modes of action.
Treatment of malaria with herbs has been in existence long before the arrival of western drugs [8]. Medicinal plants researched to date were usually selected on the basis of their traditional use and reputation for efficacy in the treatment of malaria and other diseases. The few that are being studied scientifically are Lemon grass in many parts of Nigeria, Neem leaf and barks in Northern Nigeria and the bark of Cherry mango in Southern Nigeria which have yielded positive results in reducing parasitemia [8].
Plasmodium like other parasites such as trypanosome which posses enzyme systems as sialidases, phospholipases responsible for some of the clinical symptoms like anemia in trypanosomiasis is also observed in malaria. Anemia has long been established as the main clinical feature in human and animal malaria [9-11]. Anemia occurring intra-vascularly at the beginning of the acute phase of trypanosomiasis in infected animal [12] has been attributed in part, to the cleavage of sialic acids from red blood cells’ surface by a trypanosomal enzyme Sialidase [13].
Sialic acids are a family of carboxylated saccharides containing nine (9) carbon atoms and are negatively charged. Sialic acid as a terminal saccharide residue on cell surface glycol-conjugates plays important role in a variety of biological processes [14]. They are widely distributed in nature as components of the oligosaccharide units in mucins, cellular and fluid glycoproteins, gangliosides, milk oligosaccharides and certain microbial polymers [15-17].
The erythrocyte membrane of human and animal species contains high concentration of sialic acids [18,19]. Red blood cell-sialic (RBC-sialic) acids are among the first molecules encountered by other cells or by compounds coming in contact with the cell. Sialic acid occurs on the RBC, masking galactosyl residue since galactose is the immediate sugar juxtaposed to sialic acid on the cell membrance [14]. Removal of sialic acids by the action of sialidase from microorganisms results in the destruction of such cells [20-24].
The present study aimed at evaluating Sialic acid profiles of mice infected with Plasmodium berghei and treated with methanolic extracts of Azadirachta indica as this may give an insight into the mode of action of this extracts on key pathogenic enzyme systems and their possible roles in mediating anemic conditions typical of the malaria infection
Albino mice (20-25g) free from infection, were obtained from the animal house facility of the department of Pharmacology and Clinical Pharmacy, Ahmadu Bello University, Zaria, Nigeria. They were acclimatized for a week were maintained in a well ventilated room, with temperature of 25 ± 1°C and fed on excel feeds and water ad libitum.
Malaria parasite (Plasmodium berghei) was obtained from Prof. A.J Nok (MFR) from the Kuvin Medical Centre, Hebrew University of Jerusalem Ein-keren, Israel. The strain was maintainedduring this study in the laboratory by serial blood passage from mouse to mouse.
Stem bark, seeds and leaves of Azadirachta indica (Neem) were collected from National Research Institute for Chemical Technology, Zaria and were identified at the Herbarium unit, Biological science Department, Ahmadu Bello University, Zaria as Azadirachta indica A. Juss, with Voucher number 900151.
The stem bark and leaf were cut into smaller pieces and dried in the laboratory and dried seeds collected, were processed and the kernel removed. The respective samples were ground to fine powder and 100 g of each sample was extracted with 500 ml methanol and left for 72 hours at room temperature in an orbital shaker. The different extracts were filtered and concentrated under vacuum in a rotatory evaporator. The extracts were kept in tightly closed bottles at 4 OC in a refrigerator until use.
A donor mouse with a rising parasitemia of 20 % was sacrificed and its blood was collected in heparinized syringe and diluted in phosphate buffered saline to108 parasitized erythrocytes/ml. The infection of mice was initiated by needle passage of the Plasmodium berghei parasite preparation from the donor mouse to healthy test mice via an intraperitoneal route [25]. Each mouse received 0.2 ml (2 x 107 parasitized erythrocytes) of dilute infected whole blood. Parasitemia was monitored by microscopic Giemsa-stained thin blood smears. The number of parasitized erythrocytes in each of the ten such fields was counted thrice and the average was computed to give the level of parasitemia of each mouse [25,26].
Thirty two mice were used and they were divided into eight groups (A, B, C, D, E, F, G and H) of four each. The mice were infected with 0.2ml of diluted (108 parasitized erythrocytes/ml) infected blood each intraperitoneally (i.p) except those in group H that form normal control group [25,27].
Treatment of mice in experimental groups A to F commenced 2 h after infection at a dose of 10mg/kg body weight and treatment was repeated daily with the same dose for 72 h. The Neem extracts were dissolved in dimethyl sulfoxide (DMSO) and 13% Ethanol.
Group A – Control (infected with no treatment)
Group B – Treated with choloroquine (CHQ) (10mg/kg)
Group C – Treated with Artheeter (ATM) (10mg/kg)
Group D – Treated with Quinine (QUI) (10mg/kg)
Group E – Treated with Neem leaf extract (NLE) (10mg/kg)
Group F – Treated with Neem stem bark extract (NBE) (10mg/kg)
Group G – Treated with Neem seed extract (NSE) (10mg/kg)
Group H – Not infected (normal control treated with DMSO)
Twenty four hours after the last treatment (96h post-infection) blood smears from all animals were prepared and stained with Giemsa stain. Parasitemia level was determined microscopically by counting 10 fields of approximately 100 erythrocytes per field. The difference between the mean values of the experimental group was calculated and expressed as percent Parasitemia inhibition, using the following equation:
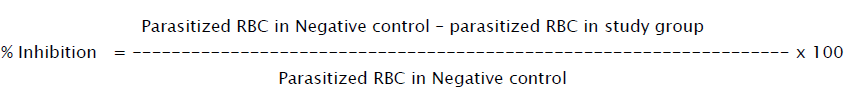
Ghost cells were prepared as described previouslyby Dodge et al [28] and sialic acid profile was determined using thePeriodate-thiobarbituric acid (TBA) Assay for Free Sialic acids Amminoff [29].The free sialic acid concentration in the serum samples collected was determined by the TBA assay [30].
The Bound sialic acid concentration in the serum was determined using 100 μl of serum incubated with 20 μl of 0.1 M HCl for 3 hours to liberate the bound sialic acid. The quantified sialic acid is known as total sialic acid ( [SA] T). The difference between the total and free sialic acid represents the bound sialic acids.
[SA] T = [SA] B + [SA] F.
Sialic acid on red cells was determined as previously described.About 100 μl of the washed erythrocyte ghosts was incubated with 100 μl of 0.1 M HCl at 80oC for 1hr to liberate the membrane bound sialic acid [31]. The liberated sialic acids concentration was determined by the TBA method.
Groups of mice treatedrespectively with 10 mg/kg/day(i.p) of three different neem extracts (leaf, stem bark and seed kernel) showed lower parasitemia bouts compared to untreated infected mice (Figure.1a and b) indicating a significant antimalarial activity of Azadirachta indica extracts against Plasmodium berghei. The stem bark extract was most effective in suppressing parasitemia levels. However, the neem seed extract was quite toxic and resulted in the death of mice within 6 days post treatment. The neem stem bark and the neem leaf extracts recorded about 55.83% and 50.74% parasitemia reduction respectively (Figure 1).
The standard drugs recorded 85.49%, 73.39 % and 29.85% for arthemeter (ATM), quinine (QUI) and chloroquine (CHQ) respectively. Chloroquine failed to suppress parasitemia like the other standard antimalarials due to the parasite’s inherent resistance to chloroquine.
Key: NLE- Neem leaf extracts, NBE – Neem stem bark extract, NSE- Neem seed extract,ATM- Arteether, QUI- Quinine, and CHQChloroquine
Also, we observed an increase in the level of free sialic acids in the serum of infected mice as infection progresses (figure 2). This also corresponds with the observed reduction in PCV profiles during infection (figure 3).
The erythrocyte surface sialic acid (RBC-SA) at different level of parasitemia showed a gradual decrease in the level of mean RBCSA( figure 4). There was no significant difference (p<0.05) in the level of bound serum sialic acid during infection as there was a gradual decrease in the bound sialic acid (figure 5).
In all the experimental groups, free serum sialic acid and RBC-SA varied from one treatment to the other (figure 6 and figure 7)There was a significant difference (p≤0.05) between the values recorded for the treated samples when compared to the values of infected non treated mice (figure 6 and figure 7). The RBC-SA (erythrocyte sialic acid from ghost cells) values in the treated mice were significantly lower than the values recorded for the normal (non-infected) mice (It is figure 7)but higher when compared to the infected non treated mice.
There was also an increase in the free serum sialic acid of the treated mice compared to the values of normal mice (figure 6). A similar data was also obtained in the sialic acid profiles of the brain homogenates of treated mice (figure 8). The values recorded in the brain homogenates were higher than those obtained from the serum samples. The serum free sialic acid in the survivors treated were almost normalized (figure 9) when compared to experimental controls except for the value obtained for chloroquine treated mice which wassignificantly higher (p≤0.05).
The terminal sialic acid from the sugar residues and glycoproteins is cleaved by the enzymesailidase, and the detection of sialidase rests on the assay of free sialic acid split from asubstrate [32]. It was observed during this study that Plasmodium berghei (ANKA strain) produced sialidase in vitro. The increase in the level of free serum sialic acid accompanied by little decrease in the level of bound serum sialic acid and a significant decrease in the erythrocyte surface sialic acid suggests that, the RBC is the principal source of the released sialic acids.
A decrease in packed cell volume, an index of anaemia [31] was observed in the Plasmodium berghei infected mice. The decrease in PCV corresponds to a reduction in the RBC-sialic acid, which in part was reported to cause anaemia as a result of increased erythrophagocytosis and reduction in erythrocyte half-life [23,33-35].
Free serum sialic acid was very high in mice with high parasitemia. It is known that sialidase cleaves sialic acid from the erythrocytes of infected animals [36,31]. The cleavage could explain why infected animals had very high free serum sialic acids and relatively low erythrocyte surface sialic acids.
The extracts of the stem bark and leaf of neem suppressed parasitemia by 55.83 % and 50.74 % respectively during the primary antimalarial assessment and also, the net effect of treatment with the extracts of this plant was the normalization of sialic acid profiles which further implicates a potential action on the enzyme sialidase.