ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
A. Murugan1, S. Saravana Babu2 N. Vijayakumar3, and A. Jeyashree4
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Benzene, toluene and xylene are the major pollutants in the petroleum industry leads to global concern due to toxicity. Isolating microorganisms which are acquainted polluted sites would be meaningful to eliminate the BTX completely. Among various bacterial isolates Pseudomonas putida, Bacillus sterothermophilus, Flavobacterium haparinum, Sphingomonas aromaricivorans, Burkholderica cepacia, Micrococcus, Alcaligenes, and Aeromonas, known to be common in all the sites. Among these Pseudomonas putida Species found to be predominant in all the samples and degrading BTX to a greater extent. Among the various organisms used P. putida was able to degrade the maximum amount of 0.52mg/l/hr benzene when these substrates were used as single substrate. But toluene and xylene were degraded at the value of 0.42 mg/l/hr and 0.30 mg/l/hr respectively. There was a slight increase in the rate of degradation when these substrates used as dual mixer (BT, TX and BX). The BT was removed at 0.45 mg/l/hr and 0.60mg/l/hr respectively. It was also different when they were used as mixed substrates (0.4mg/l/hr, 0.5mg/l/hr and 0.21mg/l/hr of benzene, toluene and xylene respectively). Exceptionally the maximum of 0.72mg/l/hr of toluene was degraded by Micrococcus lutes when it was used as dual substrate with benzene.
Keywords |
| Efficiency; Biodegradation; BTX; Industrial waste water |
INTRODUCTION |
| The accumulation of aromatic hydrocarbons and their intermediates causes detrimental effect on the different environment. During the last few decades we have contaminated our air, water and land with variety of waste products on which depends life itself. The most prominent chemical groups of organic contaminants in the water bodies are fuel hydrocarbons, polynuclear aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), chlorinated aromatic compounds, detergents and pesticides. Man-made oil spills are found to be disastrous because they introduce huge quantities of crude petroleum on ocean surface. It was known that 7× 108 gal/year of crude oil was put in to sea world wide. |
| Among various fractions released in to the environment low water-soluble components like Benzene, Toluene, and Xylenes (BTX) are major concern [1]. BTX are the major pollutants in the petroleum industry and their release in to the environment leads to global concern. Because these compounds always occur as mixtures in the environment and is difficult to remove completely. Even though their presence in the ground water is very less; their exposure can be persistent over long term. It must be removed from the environment before it gets accumulated in to the biological system. The disposal of industrial solid waste is another major problem in the treatment process. |
| Biological method is considered a cheap and most economical one [2]. Bioremediation has been substituted with conventional treatment methods remove BTX from domestic and industrial waste water for many years. Population dynamics changes according to the availability of the substrate. Hence careful isolation of microbial population from the polluted site will help in the removal of BTX available at different proportion. Microorganism has been used to remove organic matter and toxic chemicals from domestic and industrial waste discharged for many years. Careful manipulation of microorganisms acquainted in the polluted sites will be much easier for bioremediation of BTX than using microorganism isolated from different environments [3]. |
II. MATERIALS AND METHODS |
| In order to isolate bacterial populations degrading Benzene, Toluene, Xylene (BTX), the respective substrates with AR grade and commercial AR grade of catechol were procured from E-Merk, Mumbai. |
II. A. SAMPLING |
| Waste water and sediment samples were collected from contaminated site with organic hydrocarbons using sterile techniques randomly every month from each site. Immediately after the collection the samples were, kept in the ice boxes and brought to the laboratory within six hours. Total viable count and BTX degrader present in the samples were analysed using standard methods [4]. |
II. B. PHYSICO-CHEMICAL PROPERTIES |
| The physiochemical properties of the effluents were analysed by following the procedure of APHA [5]. Samples for physiochemical and bacteriological analyses were preserved at 50ºC. All the bacteriological and physicochemical analyses were done within 24 hrs from the sample collection. |
II. C. ISOLATION AND ENUMERATION OF BTX DEGRADER |
| One ml of waste water sample collected from different polluted sites was dissolved in 99 ml of sterile distilled water, and from this master dilution, it was diluted serially up to 10-8. The diluted samples were plated over the Minimal Salt Agar medium supplemented with BTX at the concentration of 0.1%. The seeded plates were sealed with parafilm to avoid volatilization of aromatic hydrocarbons and incubated at 30 ºC for 48 hours. |
II. D. ENUMERATION OF TVC AND HYDROCARBONOCLASTIC BACTERIA |
| To determine the total viable count the effluent samples were collected in sterile plastic bags and transported to the laboratory with ice cold container. Bacteria were enumerated as Colony Forming Units (CFU) employing the standard pour plate technique. Plate count agar medium (HI Media Laboratories, Mumbai, India) was used to enumerate TVC. Waste water samples collected in sterile containers were serially diluted in saline (0.85% of NaCl). The dilutions were plated over respective medium and the plates were incubated at 30ºC. After 3-5 days of incubation, colonies were counted using a colony counter. Total viable count was expressed as CFU number per 100 ml. |
II. E CHEMOTAXIS PATTERN OF BTX DEGRADER |
| Petri plates containing 2% low-melting temperature agarose dissolved in chemotaxis buffer (40 mM potassium phosphate [pH 7.0], 0.05% glycerol, 10 mM EDTA), 10% (vol/vol) of BTX as individual substrate, and it was also added at different combinations. A few crystals of comassive blue were added to create contrast in the medium. A drop (10 ml) of the molden agarose mixture was placed on a microscope slide, and a cover slip supported by two plastic strips was then placed on the top to form a chamber. Cells were harvested in midlog phase suspended in chemotaxis buffer to an OD at 660 nm approximately 0.7 and flooded into the chamber to surround the agarose plug. The seeded plates were incubated at 30oC for 48 hours after the plates were sealed with parafilm. After the incubation the plates were observed for the presence of zone of growth around the wells. Growth was measured in terms of zone of growth in millimeter and it reflects the ability of microorganisms to able to utilize substrates. |
II. F. EFFICIENCY OF BTX DEGRADATION AS A SINGLE SUBSTRATE |
| With the objective of degrading the maximum amount of BTX, the minimal medium was supplemented with the BTX at 600μl, 200μl and 100μl respectively. This setup was air sparging and was seeded with bacterial population with a density of 105 CFU/ml. The seeded flasks were incubated at 30 °C for a period of 48 hours. |
II. G. EFFICIENCY OF BTX DEGRADER AS MIXED SUBSTRATE |
| A screw cap bottle with Teflon lid was used in the present study to assess the rate of BTX degradation. With the help of a sterile syringe, samples were removed from the bottle. There are three sets of experiment were conducted to analysis the effect of BTX when they were available as mixture. In the first set benzene, toluene and xylene were added into the 200 ml of MSB at 250mg/l concentrations individually in separate containers. 1 ml of overnight broth culture obtained from Pseudomonas putida, Micrococcus lutes, Alcaligenes eutorphus and Bacillus stearothermophilus was added to the above set up and kept in a shaker at 200 rpm for a period of 18 hours. Ten ml of culture was withdrawn periodically for a period of 18 hours of which 5 ml of culture was used to check the cell density at 600 nm and the remaining 5 ml of culture was used to measure the amount of BTX and catechol accumulated. Second set of experiments were conducted with substrates were mixed with the combination of BT, BX, and TX at the concentration of 250 mg/l each substrate. Similarly third set of experiment was designed to add MSB medium with BTX as mixed substrates at 250mg/l concentration each. |
II. RESULTS AND DISCUSSION |
III. A. PHYSICO-CHEMICAL PROPERTIES OF DIFFERENT SAMPLES COLLECTED FROM POLLUTED SITES |
| Evaluation of the different physico-chemical properties tested with the samples collected from five different locations showed different parameters which are higher in their value than actual permissible limits (data not shown). |
III. B. ISOLATION AND ENUMERATION OF BTX DEGRADER |
| In order to isolate total BTX degrader, different sources which are highly contaminated with BTX have been chosen, maximum number (78×108 CFU/ml) of total viable count (TVC) and benzene degrader (25×104 CFU/ml) with the sample collected from Harbour. The sample collected from Phyto-chemical industry recorded a higher number (63×103 CFU/ml) of Toluene degrader. Xylene degraders were found to be as high as 65×102 CFU/ml in the sample collected from oil refinery effluent. Very interestingly, the samples collected from petrol bunk areas exhibited significantly less number of TVC and BTX degrader compared with other four different samples (data not shown). To achieve successful removal of BTX from polluted sites, using microorganism it is necessary to understand the microbial characteristics, such as the indigenous microbial community and its biodegradability within the area [6]. Microorganisms isolated from polluted sites are able to use BTX as the sole carbon source as well as energy substrate through TOL pathway. Similarly ZoBell [7] identified over 100 microbial species from 30 genera that could degrade hydrocarbon. The existence of BTX degrader is known and they are distributed widely. The ubiquity of soil bacteria, capable of degrading BTX was first demonstrated in 1928 and 146 out of 245 contaminated soil samples contained bacteria capable of metabolizing hydrocarbons [8]. |
III. C. CHARACTERIZATION AND DISTRIBUTION OF BTX DEGRADER |
| Owing to identify the efficient BTX degrader, all the isolates obtained from different samples were characterized. The organisms identified were Pseudomonas putida, Micrococcus lutes, Bacillus stearothermophilus, Alcaligenes eutrophus, Klebsiella Sps, Flavobacterium ferrugineum, Acinetobacter radiodurans, Mycobacterium vaccae, Aeromonas salmonicida, and Enterobacter ludwigii. Among these organisms Pseudomonas putida are found to be a predominant species in all the samples collected and it comprises about 24%. Maximum of 18 colonies were found in the Harbour sample. This was followed by the automobile washing area, where 14 CFU/ml of P. putida were isolated. Among the different samples, the samples collected from petrol bunk and effluent from oil refinery were found to have less number of colonies (11 CFU/ml). Other than P. putida were found to be moderate number ranging from 12 to 14 colonies. The second most predominant species isolated was A. eutrophus, which comprised 19%. Acinetobacter radiodurans, Aeromonas salmonicida, and E. ludwigii were found to be less in the number in all the samples (Table 1 and Figures 1 to 5). |
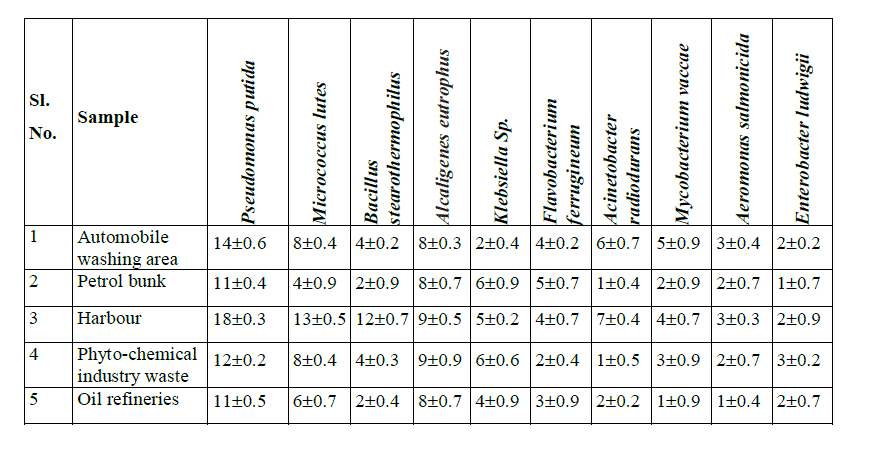 |
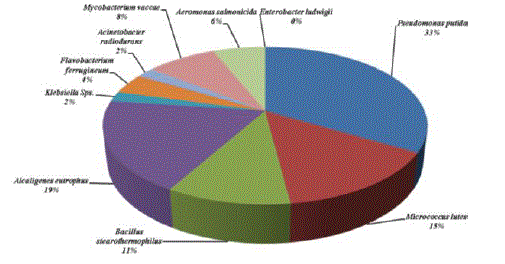 |
 |
| The present investigation also coincides with experiment conducted by Stapleton et al., [9]. They have identified Pseudomonas sturzeri, Pseudomonas aeruginosa, Bacillus subtilis, Flavobacterium haparinum, Sphingomonas aromaricivorans, Burkholderica cepacia, Micrococcus, Alcaligenes, and Aeromonas, as common population found in the contaminated sites. Of these, Pseudomonas spp, Alcaligens spp, Acetobacter spp, and Bacillus are the predominant forms [10]. Our study known to differ from Philp et al., [11] and they have stated that the distribution of the two genera, namely Pseudomonas and Micrococcus are more predominant than any other forms. Similar findings were also reported by Akoachere et al. [12]. Atlas and Bartha [13] suggested that presence of petroleum hydrocarbons influencing the diversity, distribution and population of microorganisms in an environment. Oil-degrading bacteria isolated from contaminated areas imply that the soils have the ability to self-purify in case of major spillage. |
III. D. CHEMOTAXIS OF BTX DEGRADER |
| Chemotaxis activity of different isolates was tested with different combinations of BTX using agar diffusion method. Chemotaxis was measured in terms of zone of growth. Among the various organisms tested Pseudomonas putida was attracted by different substrate and grow well with all the possible combinations of BTX, except TX. Maximum growth zone (6.4mm) was observed with BT while minimum zone of growth (1.2mm) was observed with Enterobacter ludwigii when they were growing with T and X. The overall growth response was very much appreciable with B and T combinations followed by benzene. On the other hand, with TX and X were shown less growth of all the organisms tested. P. putida and M. lutes found efficient in sensing and swimming towards B and T. B. stearothemophilus was attracted towards X. The results indicated that these strains were able to use these substances for their growth (Table 2, Figures 6-7 and Plate 1). Parales et al. [14] stated that chemotactic cells are able to sense their growth substrate and swim efficiently towards chemicals that are present in the polluted sites. Growth of these isolates towards BTX reveals the ability to utilize these compounds. The study by Heipieper and Bont [15] also confirmed that BTX adapted bacterial strains are more tolerable to hydrocarbons. |
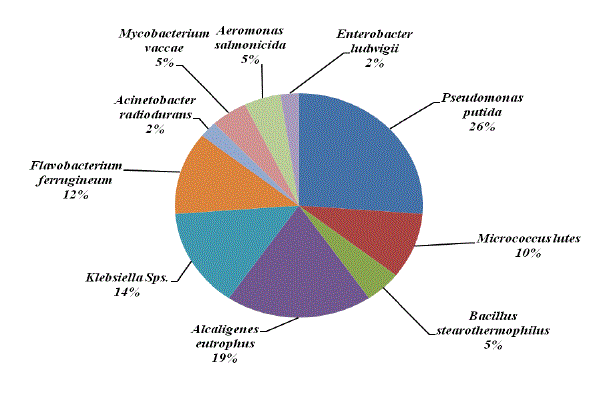 |
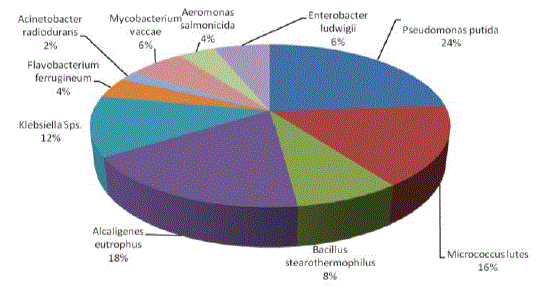 |
III. E. GROWTH PATTERN OF BTX DEGRADER |
| Studies conducted on the growth pattern of different BTX degrader on BTX showed significant results. Among the various organims tested for their ability to use BTX as substrate. P. putida found to grow well with benzene other strains. M. lutes and A. eutrophus used toluene as a growth substrate. B. stearothermophilus grow well with xylene when compared with any other strains tested. On the other hand, rest of the six isolates showed no significant pattern of growth. Venkateswaran et al. [16] group Pseudomonas species shown outstanding adaptation towards higher molecular weight crude oil and were able to degrade many of its components. M. lutes and A. eutrophus were also known to use toluene for their growth. This study clearly indicates that they also possess TOL plasmid and are able to catabolise toluene for their growth. |
III. F. EFFICIENCY OF BTX DEGRADER AT DIFFERENT COMBINATIONS OF SUBSTRATE |
| Analysing the ability to utilize the different combinations of BTX by the various organisms it was found to be significant when tested using tube dilution method. The rate of removal was significant in the individual strains when BTX was available as a single substrate. Among the various organisms used P. putida was able to degrade the maximum amount of 0.52mg/l/hr benzene. But toluene and xylene were degraded at the value of 0.42 mg/l/hr and 0.30 mg/l/hr respectively. It was recorded that M. lutes was found to be the second best organism to degrade BTX in the individual form. In the case of other organisms tested the degradation rate was lesser when it was compared with that of P. putida. When it was tested with dual mixer of substrate consisting of BT, TX and BX, the rate of removal was very much significant with BT than the other two combinations. The BT was removed by the P. putida at 0.45 mg/l/hr and 0.60mg/l/hr of benzene and toluene respectively. |
| The rates of BTX degradation decreased when it was used as BTX mixer. P. putida was able to remove only 0.4mg/l/hr, 0.5mg/l/hr and 0.21mg/l/hr of benzene, toluene and Xylene respectively. The maximum of 0.72mg/l/hr of toluene was degraded by Micrococcus lutes when it was used as dual substrate with benzene. Similarly the removal rate was significantly high (0.56mg/l/hr) when they were used as mixer (BTX). On the other hand, the rate of removal was very much less in the case of Enterobacter ludwigii when it was tested with BTX as a single substrate, dual substrate as well as mixed substrate. Benzene and toluene were removed at low rates of 0.20mg/l/hr and 0.23mg/l/hr respectively. Xylene was removed at the lowest rate (0.12mg/l/hr) as mixture of substrate. |
IV. CONCLUSION |
| Bioremediation of Benzene, toluene and xylene is possible with careful manipulation of microorganisms isolated from polluted environment. Among various populations isolated Pseudomonas putida found to be predominant and it was able degrade 0.4mg/l/hr, 0.5mg/l/hr and 0.21mg/l/hr of benzene, toluene and xylene respectively when they were used as mixed substrates. Comparatively, the rate of degradation was high than the strains isolated from different environment. Hence, it is possible to remove the pollutants from the spilled area using the microorganisms isolated from same area. |
ACKNOWLEDGMENT |
| The authors wish to thank UGC, New Delhi for funding this project under Major Research Project. |
References |
|