ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
A.S. Meena*1, Rishikesh2, and R.C. Meena2
|
| Corresponding Author: A.S. Meena, |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Electrochemical studies of anionic and nonionic micelles with dyes and reductant in photogalvanic cell containing Dye-Reductant and Micelles. The photovoltages and photocurrents in photogalvanic cell containing a Rhodamine 6G-EDTA and NaLS as micelles has been determined. The photo-outputs Rhodamine 6G-EDTA-NaLS are higher than Methylene blue-EDTA-TX 100 cell. The conversion efficiency of the Rhodamine 6G-EDTA and NaLS in photogalvanic cell has been estimated to be 1.265%. The photopotential and photocurrent generated, Fill factor and storage capacity of the photogalvanic cell are determined. The effects of different parameters on electrical output of the cell are observed. The mechanism has also been proposed for the generation of the photocurrent in photogalvanic cell.
Keywords |
| micelles, photo-output, conversion efficiency, fill factor, storage capacity. |
I. INTRODUCTION |
| The solar energy is easily available, cheaper, environmental friendly source and has the potential to provide energy with almost zero emission. The novel approach for renewable sources of energy has led to an increasing interest in photogalvanic cells because of their reliable solar energy conversion and storage capacity. Photogalvanic cells are those cells in which solar energy convert into electrical energy via formation of energy rich species that exhibit the photogalvanic effect. The photogalvanic effect was first of all recognised by Rideal and Williams [1] and it was systematically studied by Rabinowitch [2-3] and then by other workers [4-9]. Some researchers [10-11] have studied on how to enhance the performance and optimum efficiency of dye sensitized solar cell for solar energy conversion. A detailed of literature survey reveals that some photogalvanic cells consisting of dye with reductant, dye with reductant and micelles for generation of electrical energy are reported [12-22]. The research in the field of photogalvanic cells is still in its infancy with respect to its viability and practical applicability and, therefore, requires thorough exploration to increase the conversion efficiency and storage capacity by selecting a suitable redox couple, dye and micelles. Therefore, the present work is the effort to observe the electrochemical studies of anionic and nonionic micelles with dyes and reductant in photogalvanic cell. |
II. METHODOLOGY |
| Rhodamine 6G (MERCK), Methylene blue (MERCK), EDTA (MERCK), NaLs (LOBA), TX 100 (MERCK) and NaOH (MERCK) are used with purification in present work. All the solutions are prepared in doubly distilled water and the stock solutions of all chemicals are prepared by direct weighing and are kept in coloured container to protect them from the light. The solution is bubbled with prepurified nitrogen gas for nearly twenty minutes to remove dissolved oxygen. Solutions of dye, reductant, micelles and sodium hydroxide are taken in an H-type glass tube. A platinum electrode (1.0 x 1.0 cm2) is immersed into one arm of H-tube and a saturated calomel electrode [(SCE- Hg2Cl2) and cell notation the electrode is written as: (Cl – (4M)/ Hg2Cl2 (S)/ Hg (l)/ Pt)] is kept in the other. The whole cell is first placed in dark till a stable potential is obtained and then, the arm containing the SCE is kept in the dark and the platinum electrode is exposed to a 200.0 W tungsten lamp. A water-filter is used to cut off infrared radiations. The photochemical bleaching of dyes (Rhodamine 6G and Methylene blue) are studied potentiometrically. A digital pH meter (Systronics Model-335) and a microammeter (Ruttonsha Simpson) are used to measure the potential and current generated by the cell, respectively. The current–voltage characteristics of photogalvanic cell have been studied by applying an external load with the help of a carbon pot (log 470 K) connected in the circuit through a key to have close circuit and open circuit device. The experimental set-up of photogalvanic cell is given in Figure 1. The effect of variation of different parameters has also been observed. The rate of change in potential after removing the source of illumination is 0.85mVmin-1 in Rhodamine 6G-EDTA-NaLS cell; therefore, the system may be used in photogalvanic cell more successfully than the Methylene blue-EDTA-TX 100 cell. |
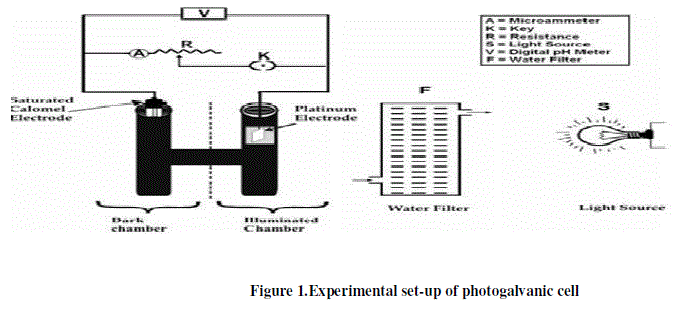 |
III. RESULTS AND DISCUSSION |
| A. Photopotential of Rhodamine 6G-EDTA-NaLS cell |
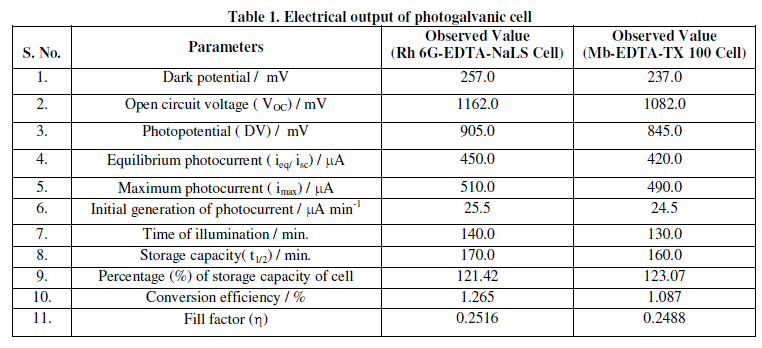
| The photopotential of Rhodamine 6G- EDTA-NaLS cell is measured at different pH values and maximum photopotential is found at pH 12.40. All the subsequent measurements are made at this pH value. The variation of photopotential with time for this cell is shown in Figure 2. As can be seen from the figure that the photopotential increase upon illumination to a value of 1162.0 mV in about 140.0 minutes and remains constant on further illumination. When the light is switched-off, the cell does not regains its original potential; thereby, showing that the cell is not perfectly reversible. |
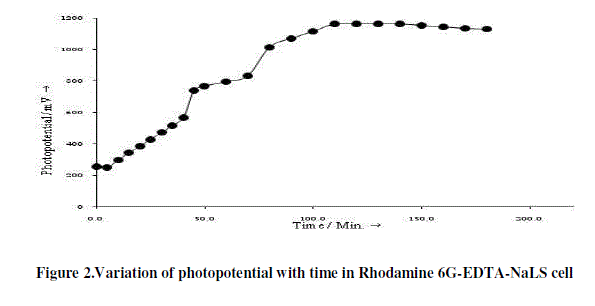 |
| B. Photopotential of Methylene blue-EDTA-TX 100 cell |
| The photopotential of Methylene blue-EDTA-TX 100 cell is measured at different pH values and maximum photopotential is found at pH 11.60. All the subsequent measurements are made at this pH value. The variation of photopotential with time for this cell is shown in Figure 3. As can be seen from the figure that the photopotential increase upon illumination to a value of 1082.0 mV in about 130.0 minutes and remains constant on further illumination. When the light is switched-off, the cell does not regains its original potential; thereby, showing that the cell is not perfectly reversible. The observed photopotentials and photocurrents in Methylene blue-EDTA-TX 100 cell are comparable less than that of the Rhodamine 6G-EDTA-NaLS cell (Table 1). |
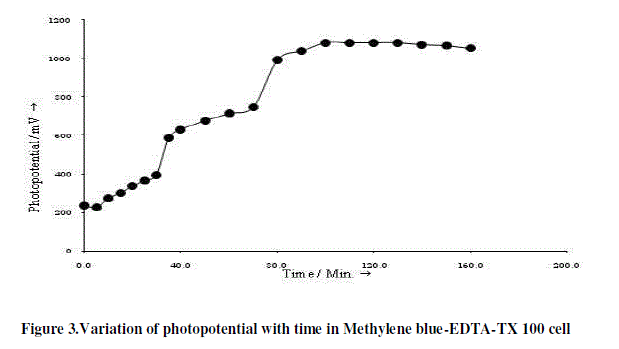 |
 |
| [Rhodamine 6G] = 2.59 x 10-5 mol L-1; Light Intensity = 10.4 mW cm-2; [EDTA] = 1.44 x 10 -3 mol L-1; Tempt. = 303 K; [NaLS] = 1.14 x 10-3 mol L-1; pH = 12.40 |
| [Methylene blue] = 2.04 x 10-5 mol L-1; Light Intensity = 10.4 mW cm-2; [EDTA] = 1.20 x 10 -3 mol L-1; Tempt. = 303 K; [TX 100] = 0.84 x 10-3 mol L-1; pH = 11.60 |
| C. Photocurrent of photogalvanic cell |
| The photo induced short circuit currents of Rhodamine 6G-EDTA-NaLS cell and Methylene blue-EDTA-TX 100 cell in photogalvanic cells are shown in Figure 4 and 5, respectively. On illumination, maximum photocurrents 490.0 μA is obtained in 130.0 minutes in Methylene blue-EDTA-TX 100 cell and 510.0 μA in 140.0 minutes in Rhodamine 6GEDTA- NaLS cell. The Rhodamine 6G-EDTA-NaLS cell takes much smaller time than Methylene blue-EDTA-TX 100 cell. The trend in short circuit photocurrents of Rhodamine 6G-EDTA-NalS cell is much better than Methylene blue- EDTA-TX 100 cell (Table 1). |
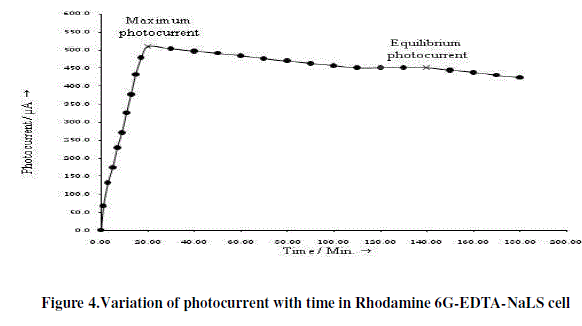 |
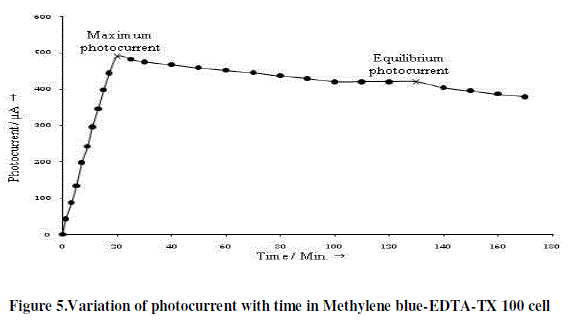 |
| D. Conversion efficiency of photogalvanic cell |
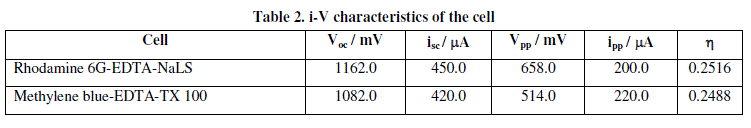
| Conversion efficiency is the important characteristics of any photogalvanic cell. The i-V characteristics of Rhodamine 6G-EDTA-NaLS cell and Methylene blue-EDTA-TX 100 cell in photogalvanic cells have been investigated to estimate the power conversion efficiency of the cell. The maximum possible power output from the cell can be obtained from the rectangle of maximum area, which can be drawn under i-V curve. The power points (a point on the curve, where the product of potential and current was maximum) in i-V curves are determined and their fill factors and conversion efficiency are also calculated by using the formula. |
 |
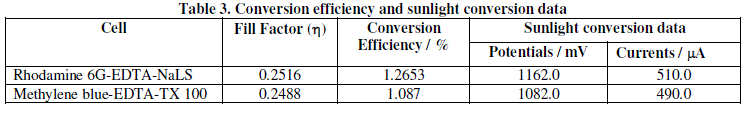
| Observed data of fill factor for these two cells are summarized in Table 2. The conversion efficiency of the Rhodamine 6G-EDTA-NaLS photogalvanic cell has been calculated to be 1.265% and that of Methylene blue-EDTA-TX 100 photogalvanic cell has been calculated to be 1.087%. The conversion efficiency and sunlight conversion data for these two cells are reported in Table 3. |
 |
| On the basis of these observations data, the higher conversion efficiency is found in Rhodamine 6G-EDTA-NaLS photogalvanic cell. |
| E. Performance of the cell |
| The performance of the photogalvanic cell is observed by applying an external load (necessary to have current at power point) after terminating the illumination as soon as the potential reaches a constant value. The performance is determined in terms of t1/2, i.e., the time required in the fall of the output (power) to its half at power point in dark. The performance of cells and t1/2 are summarized in the Table 4. |
 |
| On the basis of the observed results, the Rhodamine 6G-EDTA-NaLS is the more efficient photogalvanic cell from power generation and performance point of view. |
IV. MECHANISM |
| On the basis of these observations, a mechanism is suggested for the generation of photocurrent in the photogalvanic cell as: |
| A. Illuminated chamber |
 |
| Where Dye, Dye*, Dye-, R and R+ are the dye, excited form of dye, semi or leuco form of dye, reductant and oxidized form of the reductant, respectively. |
V. CONCLUSION |
| Photogalvanic cells are cheaper due to the use of a dye, reductant and micelles, which are lower in cost and used in minute quantities of dye, reductant and micelles. On the basis of results in the present study, it is concluded that photogalvanic cells are better option for solar energy conversion and storage. Also this cell with better electrical output good performance and storage capacity may be used in near future. According to results of photogalvanic cell in these two cells, Rhodamine 6G-EDTA-NaLS cell is the more efficient than Methylene blue-EDTA-TX 100 cell due to the electrochemical properties of anionic micelles (NaLS) are more than nonionic micelles (TX 100). |
VI. ACKNOWLEDGEMENTS |
| The authors are grateful to The Head, Department of Chemistry, MLS University, Udaipur, Rajasthan-313001 (INDIA) for providing the necessary laboratory facilities to conduct this research work. One of authors (A.S. Meena) is thankful to Ministry of New and Renewable Energy (MNRE), Government of India, New Delhi-110003 (INDIA) for the financial assistance to this research work. |
References |
|