ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Nilay Kanti Pramanik1, M.Sarwar Alam2 and Rakesh Kumar Khandal*3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Effects of e-beam irradiation at different doses upto 600 kGy on injection moulded nylon 66 specimens have been investigated by solution viscosity measurement and Fourier Transform Infrared (FTIR) spectroscopy. The results of gel content and viscosity indicate that e-beam irradiation generates crosslinked structure among the polymer chains. The uncrosslinked sol portion of the irradiated nylon 66 was also affected and resulted in a decrease in chain length as reflected in the results obtained from viscosity measurement. From FTIR spectra analysis, it was found that crystallinity of nylon 66 was considerably affected upon e-beam irradiation. The decrease of N-H stretch absorbance value of irradiated nylon 66 in comparison to the pristine nylon 66, indicates the loss of hydrogen bonding due to irradiation which resulted in decrease in crystallinity
Keywords |
| Nylon 66, e-beam irradiation, gel content, sol content, crosslinking, crystallinity, viscosity, FTIR spectroscopy. |
INTRODUCTION |
| High energy electron beam radiation has its effect on thermoplastics in various ways. Some of them produce crosslinking structure resulting in enormous increase in molecular weight, whereas some polymer gets degraded through chain scission. Radiation induced crosslinking is one of the most important aspects of polymer irradiation because it helps to improve the mechanical and thermal properties and environmental stability of a polymer [1]. Today, electron beam radiation is widely used in wire, cable and rubber industries for the improvement of the performance of the product through crosslinking. The radiation processing technology replaces the conventional thermo-chemical process technology due to several advantages like; high throughput, better handling of materials, avoidance of the use of hazardous chemicals etc. |
| Nylon 66 is a versatile thermoplastic. It has excellent combination of strength, stiffness, toughness, lubricity and resistance to fatigue [2]. Inspite of those excellent properties, nylon 66 could not find application in high performance engineering field due to drawbacks like higher hygroscopicity, poor dimensional stability, processibility, insufficient rigidity etc [3]. |
| Thus in order to overcome those inherent drawbacks and to make nylon 66 a successful engineering plastic, radiation processing technology has been applied. |
| Effect of high energy radiation on nylon 66 has been studied by several researchers. Radiation crosslinking of nylon has been reported by various researchers, where in most of the cases extruded films, yarns or fibers have been used [4-7]. In our earlier studies we have carried out detailed investigation on high energy radiation processing on injection moulded nylon 6 and nylon 66 specimens [8-9]. In this communication, we have carried out solution viscosity study and FTIR analysis of unirradiated nylon 66 and e-beam irradiated nylon 66 in order to investigate the structural changes that occurred due to e-beam irradiation of injection moulded nylon 66 specimens. |
EXPERIMENTAL |
A. Materials |
| Nylon 66 granules, grade Zytel 101L, from DuPont, USA, were used in this study. This is a lubricated, natural nylon 66 resin for injection moulding. LR grade formic acid (90 %) from SD Fine-Chem limited, India, was used as solvent for the viscosity measurement. |
B. Sample Preparation by injection moulding |
| As nylons are very much hygroscopic in nature, before being used for injection moulding the nylon granules were dried in an oven at 80 °C for 24 hours. The dried granules were then injection molded at 270 0C into dumbell specimens using a microprocessor-based reciprocating screw-type injection-molding machine. The molded dumbell specimens were packed in a polyethylene pouch and stored in a desiccator till the irradiation experiment. The details of the injection moulding condition is given in Table 1. |
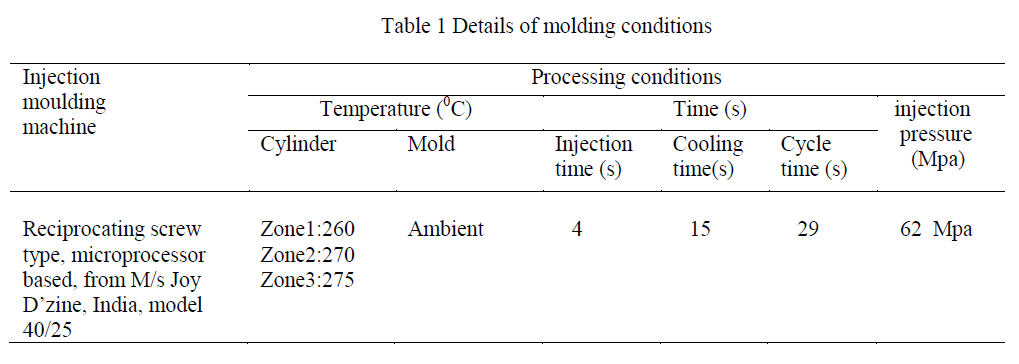 |
C. Electron beam Irradiation of the molded specimens |
| Nylon 66 dumbell specimens were irradiated by e- beam at Bhabha Atomic Research Center, India, using a 2 MeV ebeam accelerator. The irradiation was carried out in air at room temperature. The specimens were arranged in array on stainless steel trays and attached to the conveyor system which carried the trays at a speed of 3 cm/s. A blower was used to avoid overheating of the samples during irradiation. The samples had received 10 kGy e- beam dose per pass. The samples were exposed to 100, 200, 300, 400 and 600 kGy e-beam dose. After irradiation was over, the specimens were repacked in zipper polyethylene (PE) bags and stored in desiccator containing silica gel as desiccant. |
D. Characterization |
| The e-beam irradiated specimens were characterized for solution viscosity and FTIR analysis to investigate the structural changes that occurred due to e-beam irradiation. |
Viscosity study |
| Relative and inherent viscosity was determined for both pristine and e-beam irradiated nylon 66 by using Ubbelohde capillary viscometer. The viscometer size was selected to maintain the flow time of solvent above 100 s for kinetic energy corrections [10]. The test was performed as per guide lines of ASTM D 4603-9. The solution of nylon 66 was prepared by dissolving accurately weighed 0.25 g sample in 50 ml formic acid in a volumetric flask and kept for several hours at room temperature in a orbital shaker. For crosslinked nylons the solution was prepared taking the sol portion of the sample, which has been separated from the gel through solvent extraction. As the viscosity is highly dependent on the temperature, all the measurement have been done using a water bath maintained at uniform temperature 23 ± 1ºC. |
| Relative viscosity, which is the increment in the solution viscosity (η) with respect to that of the pure solvent (η0), is simply the ratio of flow time of solution (t) to that of solvent (t0). |
| Thus the relative viscosity, ηr = η/η0 =t/ t0 |
| The specific viscosity of a solution of concentration c is ηsp = (η − η0)/η0 = ηr − 1 |
| Both ηr and ηsp depend on the polymer concentration. Thus, inherent viscosity is calculated using the equation: ηinh = (ln ηr)/c |
| The intrinsic viscosity, [η], was determined using Billmeyer relation [11]. |
| [η]=0.25 (ηr -1+3lnηr)/ c |
| The equation helps to determine intrinsic viscosity through a single specific viscosity measurement [12]. The equation has been derived from Huggins and Kraemer equations ignoring the negligible higher terms. This equation is found to be valid at low polymer concentrations, and graphical extrapolation of any one of them is expected to produce more or less the same value of [η]. More over a considerable amount of time, effort, and materials can be saved in this process. Sometimes, the graphical solutions do not provide very accurate estimation of [η] [13]. |
| The intrinsic viscosity [η], which is a measure of the hydrodynamic volume of the polymer coil, is related to viscosity average molecular weight, Mv, through Mark-Houwink equation. |
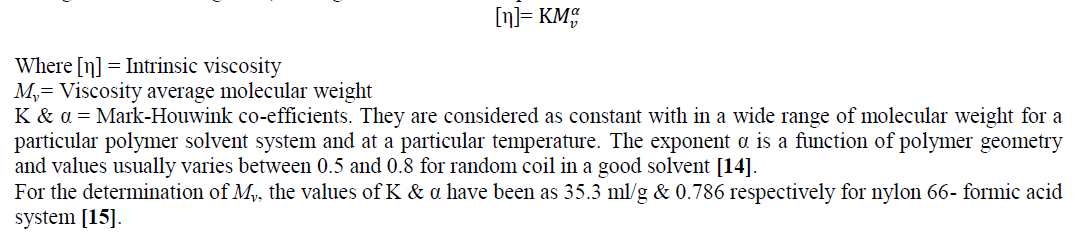 |
FTIR study |
| The FTIR spectra have been recorded in the mid-infrared spectrum region (4000-400 cm-1) using an ABB FTIR spectrophotometer (Model FTLA 2000-100). Finely ground nylon 66 and e-beam irradiated nylon 66 were mixed with dry KBR powder and pallets are prepared by compressing in a pellet-making press. The KBr discs with the polymer sample were placed in the IR cell and the spectrum was recorded. IR is a useful and highly specific tool to identify the chemical nature of the polymer and to determine its composition [16]. |
RESULTS AND DISCUSSION |
| Viscometry is the most popular method for molecular characterization of nylons. As the solution viscosity depends on molar mass and chain length of the dissolved polymer, by measuring solution viscosity the information on the molecular characterization obtained very easily. Once the relative viscosity (ηr) which is the increment of flow time of polymer solution to that of pure solvent is determined, the relevant important parameters like specific viscosity (ηsp), inherent viscosity (ηinh) and intrinsic viscosity [η] are easily calculated from it. |
| It was observed that e-beam radiation of nylon 66 produces crosslinked structure and thus insoluble in formic acid [8]. In the present study we have seen that insoluble gel formation started at 200 kGy and then the gel content increases with the increasing radiation doses (Figure 1). |
| The uncrosslinked portion of the irradiated materials which is mainly crystalline zone is soluble in formic acid. The radiation has its effect on the uncrosslinked sol portion. Hence the viscosity of these samples was determined on the sol portion, which had been separated from the gel through solvent extraction method. |
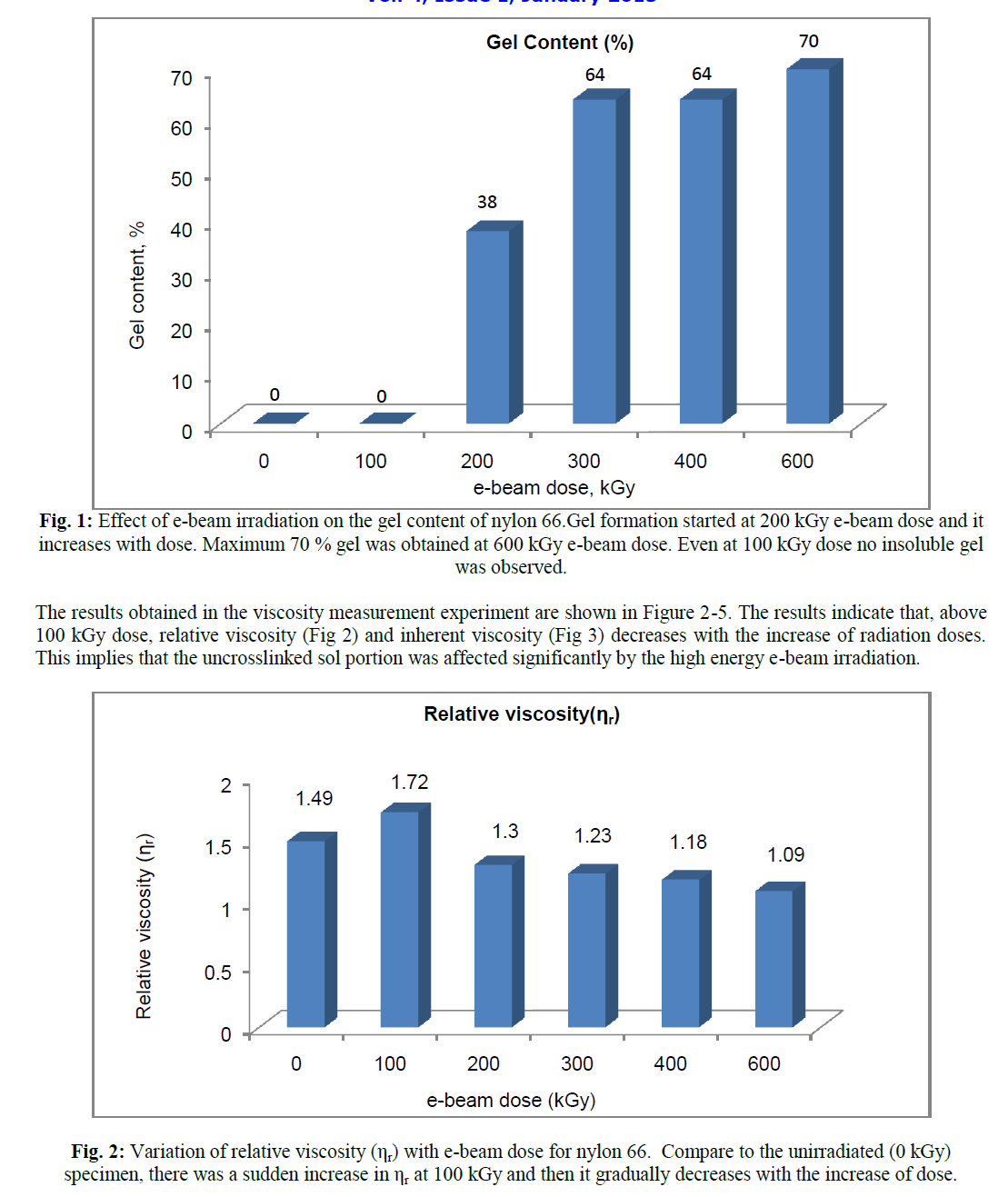 |
| Decrease in relative viscosity and inherent viscosity with the increase of radiation dose indicates, the chain length and hence the molecular weight in the uncrosslinked zone decreases as the radiation dose increases. However upto 100 kGy e-beam dose, both relative viscosity (ηr) and inherent viscosity (ηinh) was found to increase. On moving from 0 to 100 kGy, ηr increases from 1.49 to 1.72 and ηinh increases from 0.7976 dL/g to 1.0791 dL/g. |
| The results of intrinsic viscosity, [η], determined from Billmeyer relation, has shown figure 4. The variation of [η] with e-beam dose is quite similar to that of ηr & ηinh. With the increase of radiation dose, [η] showed maximum value at 100 kGy and then it decreases steadily with increasing dose. Intrinsic viscosity [η] is a measure of the hydrodynamic volume of the polymer coil. Thus decrease in intrinsic viscosity of the sol portion above 100 kGy, means decrease in hydrodynamic volume of the macromolecules. So radiation induced chain scission causes decrease in chain length which resulted in lowering the hydrodynamic radius of the chain. |
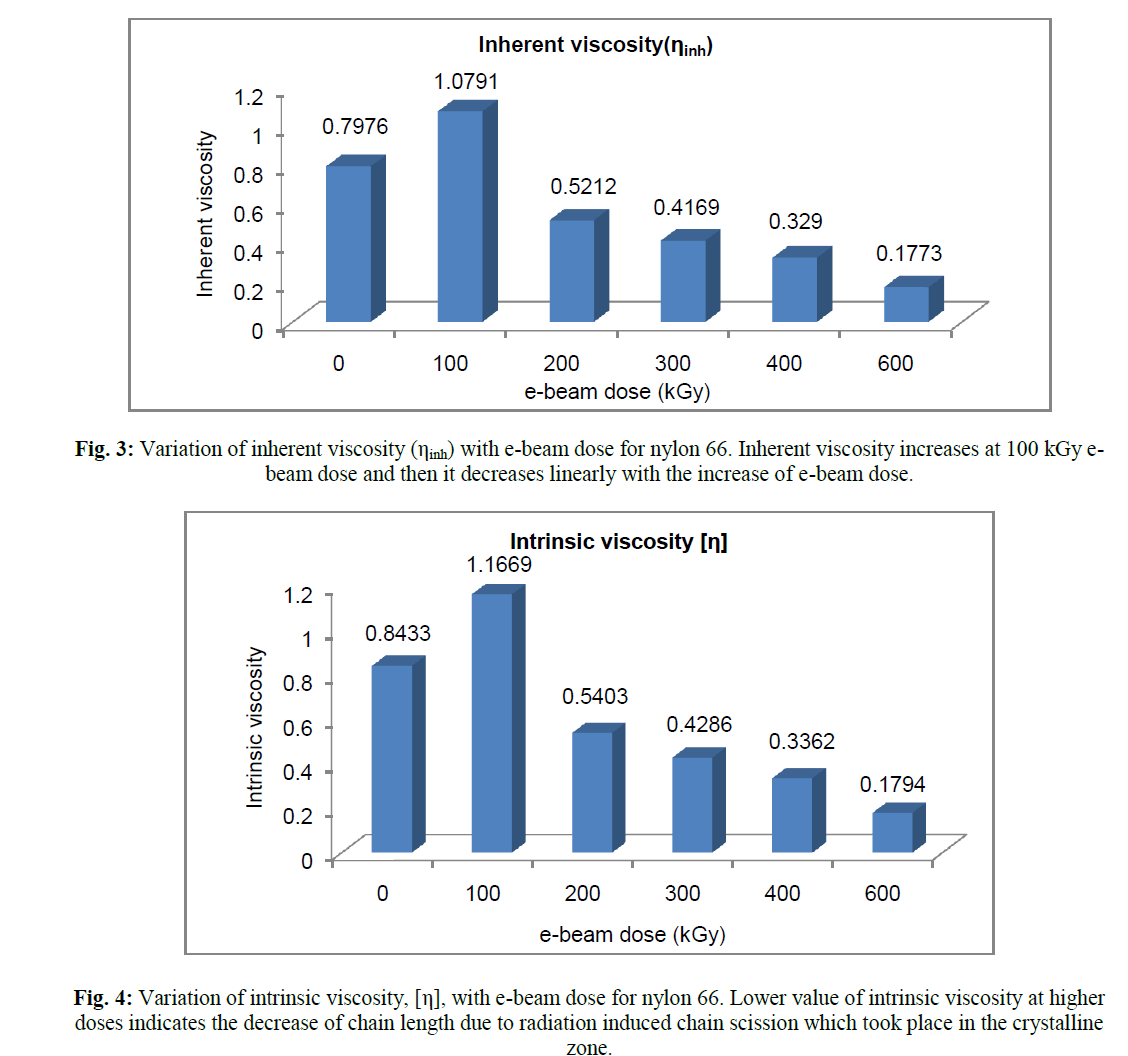 |
| The viscosity average molecular weight (Mv) determined using Mark-Houwink equation confirmed the above fact. The absorbed energy received from radiation resulted in breaking of polymer chain and thus reduced the chain length of those molecules which did not take part in crosslinking. Radiation induced crosslinking in semicystalline polymer generally takes place in the amorphous phase and in the interface between the amorphous and crystalline phases. The crystalline phase is mainly responsible for chain breaking phenomenon [17]. Crystalliniy is therefore, expected to decrease with radiation dose, because chain scission in the crystalline region would generate defects in the crystal [17]. Pramanik et al. had reported earlier that percent crystallinity of nylon 66 decreases with increasing e-beam radiation dose [8]. It is further verified by the viscosity study that chain scissioning which mainly occur in crystalline phase, increases with radiation dose. However, the observation was different for the specimen which had received 100 kGy ebeam dose. Relative viscosity (ηr), inherent viscosity (ηinh) and viscosity average molecular weight (Mv) were found to increase at 100 kGy e-beam dose. The sample did not form any insoluble gel in formic acid. Thus 100 kGy e-beam dose did not generate a strong three dimensional crosslinked network in the amorphous phase that could not be disintegrated by dissolution in formic acid. However, an increase in viscosity at this dose indicates that was certain increase in chain length due to the formation of intermolecular crosslinking; but the dose was not high enough to produce an insoluble three dimensional crosslinked structure. The above observations were confirmed by calculating viscosity average molecular weight, Mv, from intrinsic viscosity data using Mark –Houwink equation (Figure 5) where a sudden rise in viscosity average molecular weight (Mv) took place. |
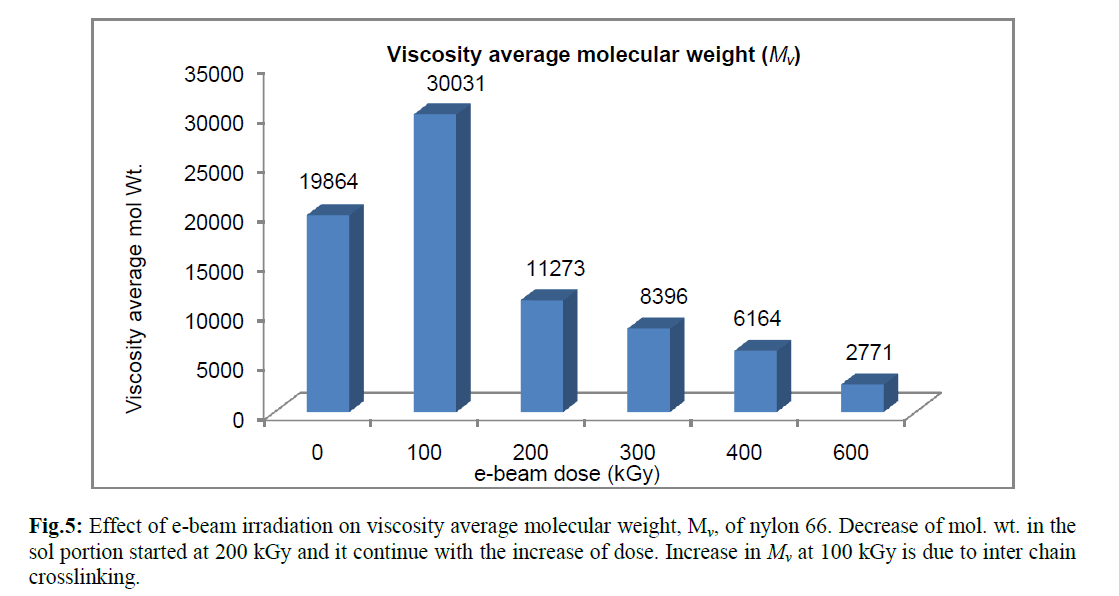 |
FTIR study |
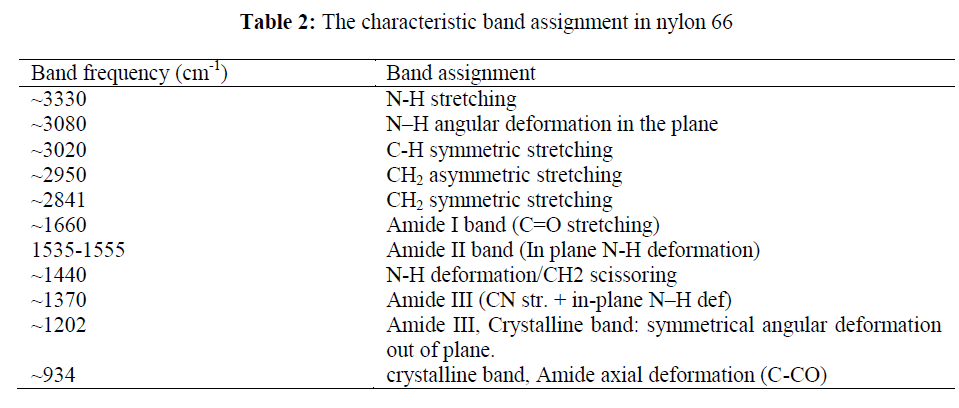
| FTIR spectroscopic technique has been widely used for identification of the basic structural units present in nylon 66 as it provides detailed information of the characteristic vibrational energies of the various groups present in the molecule. Every peak position is fundamental to molecular bonding structure or functional group present in Nylon 66. So, any shift in peak position in a spectrum directly refers to a change in bond strength or bond angle. Weakening and strengthening of a bond shifts the wave number of the corresponding absorption peak to lower and higher values, respectively. Absence of a particular absorption band relates to scission or break in the particular bonding structure. In addition to this, variation in intensity of particular peak in a spectrum correlates to the proportion of that functional group present in the material. Nylon 66 has amide (CONH) group in the repeating unit resulting in H-bond formation between C=O group in one chain to the NH group of the other chain. The H-bonding is responsible for the formation of crystalline structure in nylons. The assignments of all the fundamental bands are given in Table 2 [18]. |
 |
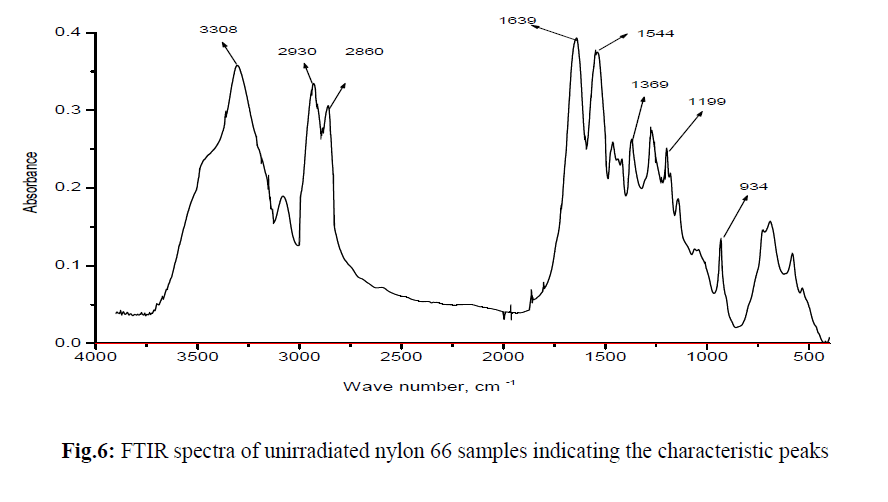
| FTIR spectrum for unirradiated nylon 66 sample is shown in Figure 6. FTIR spectra of all the e-beam irradiated nylon 66 samples are compared with that of unirradiated Nylon 66 and are shown in Figure 7. From FTIR spectra it is clear that the absorption intensity at various band position changes drastically after nylon 66 irradiated by e-beam. In the frequency range below 1500 cm−1 the observed peaks having medium/ weak intensity where as in 1700–1500 cm−1 frequency range the observed peaks are strong and are assigned for amide I and II bands. The weak and broad band observed at 1144 cm−1 has been assigned for the amorphous phase and the peaks arise due to the presence of chain defects in this phase. The other two important peaks below 1500 cm−1 are at 935 cm−1 and 1200 cm−1 which are assigned for the characteristic crystalline peaks [19, 20]. The intense amide I and II bands are observed in the frequency range 1700–1500 cm−1. C–N stretching of amide II band at ~1535 cm−1, C=O stretching vibration of amide I band at ~1635 cm−1. A very sharp peak at about ~3300 cm−1 is observed which is due to N–H stretching of the amide group present in nylon 66. As the N–H group form hydrogen bond with C=O group of the vicinal nylon chain thus the band at ~3300 cm−1 are very sensitive with the variation of hydrogen bonding [20]. |
 |
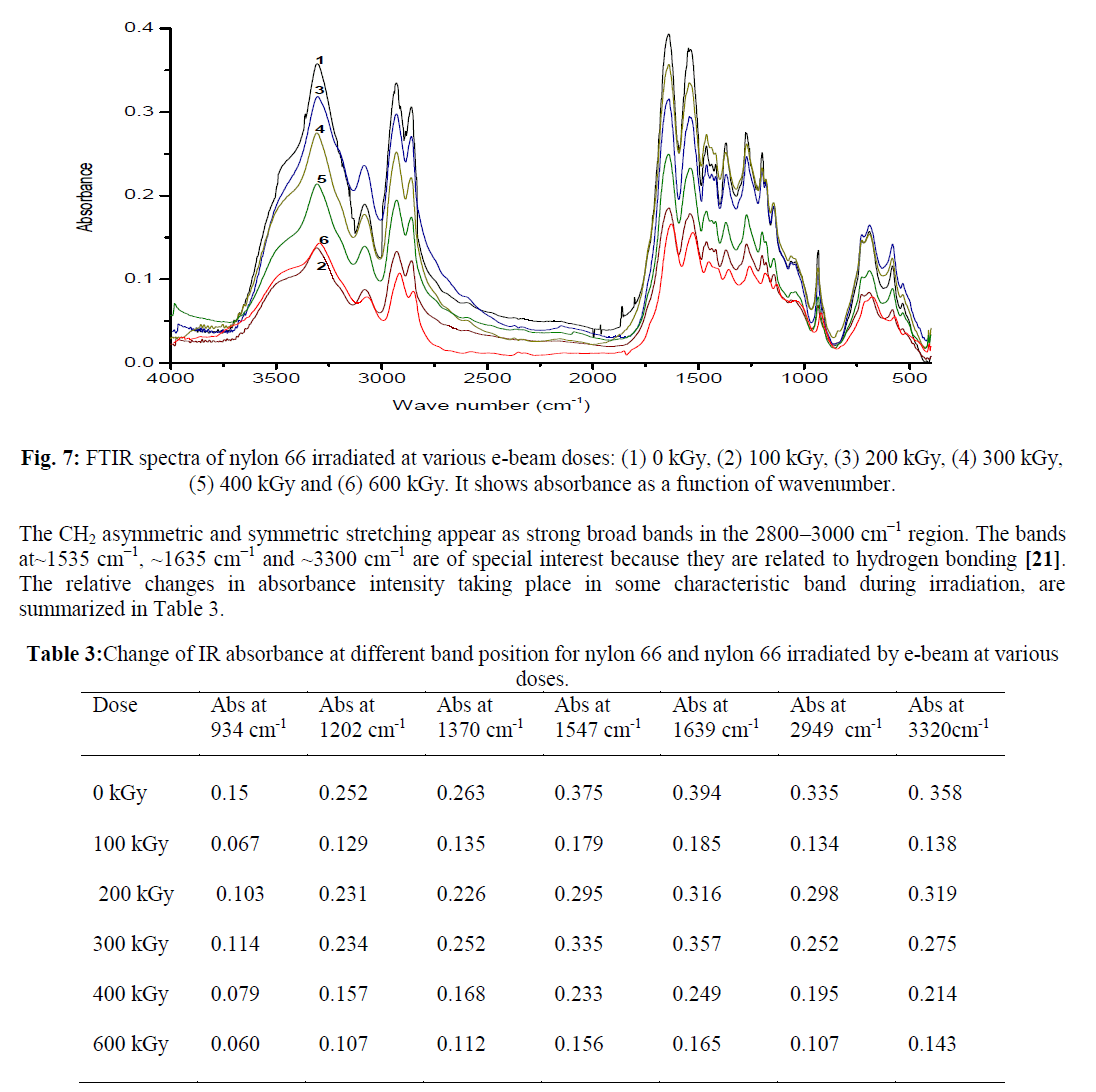 |
| When e-beam dose increased from 0 kGy to 100 kGy there was a sharp drop in absorbance intensity at ~ 3320 cm-1 from 0.358 to 0.138. This is due to loss of N–H stretching absorbance which arises due to loss of hydrogen bonding after irradiation. But at higher radiation dose, the decrease in absorbance intensity at ~ 3350 cm-1 is less which is due to the decrease in number of N-H bonds. The intensity of absorbance at ~934 cm-1 and ~1200 cm-1 ,which are assigned for the crystalline phase of nylon 66 was also found to decrease with increasing radiation dose. E-beam dosage increase from 0 to 600 kGy, the absorbance value at ~ 934 cm-1 decreases from 0.15 to 0.06 and at ~ 1202 cm-1 the absorbance value decreases from 0.252 to 0.107. This indicates that the crystalline phase of nylon 66 has been changed by e-beam irradiation. The chain scission phenomenon which predominantly occurred in crystalline phase produced crystal defect and reduced the crystallite size upon e-beam irradiation [17]. Decrease in percentage of crystallinity in nylon 66 upon e-beam irradiation has been reported by several workers [5]. We have reported in one of our communication, that nylon 66 when irradiated by electron beam radiation at 600 kGy, shows a decrease in percentage crystallinity from 40.8 % to 20.4 % [8]. |
CONCLUSIONS |
| The characterization studies on nylon 66 and e-beam irradiated nylon 66 were carried out by measuring the solution viscosity and FTIR spectroscopic technique. The viscometry study reveals that e-beam irradiation of nylon 66 resulted in the formation of insoluble crosslinked structure. The uncrosslinked portions, which are mainly from crystalline zone were also affected significantly due to e-beam irradiation. The viscosity of the uncrosslinked sol portion found to decrease with increasing radiation dose, indicates the chain length of the irradiated specimens have been reduced. The viscosity average molecular weight, Mv, determined from Mark-Houwink equation, clearly indicates that chain scission phenomenon occurred in the uncrosllinked sol part of nylon 66. FTIR study reveals that after e-beam irradiation the morphological structure of nylon 66 was changed. The decrease of crystalline absorption band intensity after irradiation indicates crystallinity of nylon 66 decreases due to irradiation. |
ACKNOWLEDGEMENT |
| The Authors acknowledge Shriram Institute for Industrial Research, New Delhi for their support and guidance in conducting the research work. |
References |
|