ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Keyur Raval1, Dhanya Sunil2, Sakshi Singhania3, Praful Deshpande4
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Biodegradable nano size biopolymers have important potential applications for administration of therapeutic molecules. The aim of the present investigation was to demonstrate the synthesis and characterization of biodegradable nanoparticles based on chitosan for encapsulation of a novel water insoluble drug 6-[3-(4-chlorophenyl)-1H-pyrazol-4-yl]-3-[(2-naphthyloxy) methyl][1,2,4]triazolo[3,4-b][1,3,4]thiadiazole (CPNT). Drug containing nanoparticles were prepared with different amounts of chitosan using ionic gelation method. The mean size and size distribution of nanoparticles were measured by dynamic laser light scattering and confirmed with Scanning Electron Microscope (SEM). The mean particle size, varied in the range of 117- 122 nm. The maximum encapsulation efficiency of 75% was encountered using drug:polymer ratio of 1:10. IC50 value of chitosan encapsulated CPNT was five times less than non-encapsulated drug and was almost 24 times less than the standard doxorubicin drug.
Keywords |
| Bioavailability, Chitosan, Nanoparticles, Encapsulation, Anticancer |
I. INTRODUCTION |
| Glioma is one of the most common intracranial tumor. Infiltration of the central nervous system by neoplastic cells in glioblastoma patients leads to neurological dysfunction and eventually to death. The different treatment modalities include micro-surgery, chemotherapy and radiotherapy. But the average survival time of patients was less than a year suggesting unsatisfactory treatment results. Carmustine is the most commonly used fat-soluble glioma chemotherapy drug through the blood-brain barrier. However, intravenous administration of Camustine, can lead to a series of serious complications such as bone marrow suppression, liver toxicity, lung fibrosis, with rather limited treatment effects. The development of new methodologies that have higher drug selectivity for cancer while simultaneously reducing the toxicity of healthy tissues is a major challenge in cancer treatment. |
| Targeted delivery of therapeutic nanoparticles in a disease-specific sustained drug release manner represents a potentially powerful and attractive technology especially when treating infiltrative brain tumors such as gliomas[1]. |
| 1,2,4-Triazole derivatives are a class of heterocyclic compounds with the advantage of broad spectrum activity, high oral availability, and toxicity [2]. These compounds possess antitumor activities along with various other medicinal properties such as antiviral, antimicrobial, antifungal and anti-tubercular. A recent report from Dhanya et al demonstrated anticancer and antioxidant activity of 6-[3-(4-chlorophenyl)-1H-pyrazol-4-yl]-3-[(2- naphthyloxy) methyl][1,2,4]triazolo[3,4-b][1,3,4]thiadiazole in HepG2 cell lines[3]. However, most triazole derivatives are water insoluble which reduces bioavailability of these drugs. |
| Considerable research efforts have been spent on oral sustained release drug delivery systems with majority of the systems being solid dosage forms at nano-scale conjugated with hydrophilic polymers [4]. Nanoencapsulation of drugs using hydrophilic carriers such as chitosanhas been employed to sustain the drug release and to reduce or eliminate the various other side-effects caused by admitting oral drug dosage[4,5]. Current research in the area of chitosan nanoparticles is on delivery of therapeutic peptide, protein, antigen, oligonucleotide and genes by intravenous, oral, and mucosal administration [5]. Chitosan is a biodegradable, biocompatible and bio-adhesive polysaccharide. It has been shown that chitosan is non-toxic and soft tissue compatible in a range of toxicity tests [6].It has been widely used in pharmaceutical research and in industry as a carrier for drug delivery and as a biomedical material [7-9]. |
| Since, CPNT is water insoluble, this research work attempts to increase its bioavailability using chitosan nanoparticles as a carrier. The manuscript compares the IC50 value of chitosan encapsulated CPNT nanoparticles with non-encapsulated CPNT drug on C6 cell lines. |
II. MATERIALS AND METHODS |
| All chemicals were purchased from Sigma Aldrich, India. The minimum degree of deacetlyation of Chitosan was 85% and it had molecular weight of 200 kD. All the experiments of nanoparticles formulation were performed in triplicates and average values are tabulated. |
| A. Drug synthesis and characterization |
| The drug, CPNT was synthesized as described by Dhanya et al. 2011) [3]. To a mixture of 2.72 g, 10 mmol of 3- methyl-b-naphthyloxy-4-amino-5-mercapto-1,2,4-triazole and 2.22 g, 10 mmol of 3-(4-chloro phenyl) pyrazole 4-carboxylic acid, and 20 ml of phosphorous oxychloride were added, and the contents were heated under reflux for 18 h. Excess phosphorous oxychloride was distilled off, and the residue was poured onto crushed ice with vigorous stirring. The resulting solid was washed with cold water, 20% sodium hydrogen carbonate solution, and recrystallized from a mixture of ethanol and dioxane (1:1 mixture). The reaction sequence is outlined in Figure 1. |
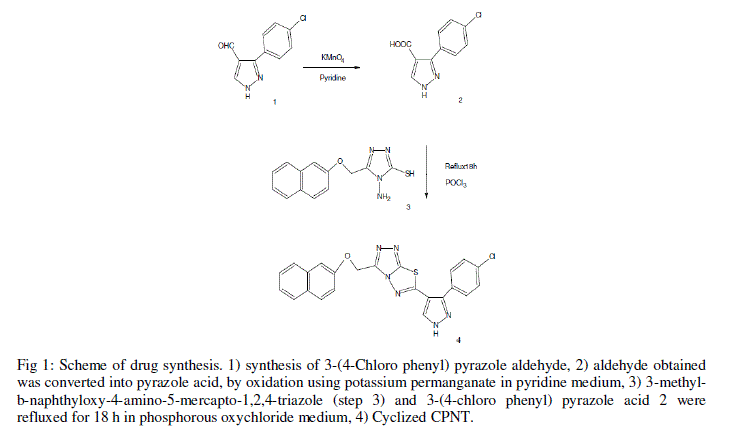 |
| Fig 1: Scheme of drug synthesis. 1) synthesis of 3-(4-Chloro phenyl) pyrazole aldehyde, 2) aldehyde obtained was converted into pyrazole acid, by oxidation using potassium permanganate in pyridine medium, 3) 3-methylb- naphthyloxy-4-amino-5-mercapto-1,2,4-triazole (step 3) and 3-(4-chloro phenyl) pyrazole acid 2 were refluxed for 18 h in phosphorous oxychloride medium, 4) Cyclized CPNT. |
| The characterization of CPNT was done using advanced spectroscopic studies and elemental analysis. Melting point was determined by open capillary method. Thin layer chromatography was conducted on 0.25·10-3 m thin silica gel plates to monitor the progress of the reaction and to check the purity of the compound. A 1:1 mixture of ethyl acetate and petroleum ether solution was used as the eluent. Visualization was made by using iodine vapors. The IR spectrum in KBr pellets was recorded on a Schimadzu FTIR 8400S spectrophotometer. 1H-NMR spectrum was recorded in deuterated dimethyl sulphoxide on an AV500 NMR spectrometer using TMS as an internal standard. The mass spectrum was recorded on a Schimadzu GCMSQP5050 mass spectrometer. The elemental analysis was done using Flash thermo 1112 series CHN analyzer. |
| B. Drug encapsulation in chitosan nanoparticles |
| The synthesized CPNT was dissolved in DMSO and then various trials were carried out to investigate amount of water required to dissolve thiadiazole without precipitation. The minimum DMSO to water ratio was found to be 1 : 9 without precipitation of CPNT. This ratio was maintained in all solutions. |
| Two ratios of drug to polymer, 1:10 and 1:20, were employed in this study. Chitosan nanoparticles were prepared as per ionotropic gelation process. Chitosan was dissolved in 0.5% acetic acid and solutions having concentration of 1.5, 1 and 0.5 mg/ml were prepared. CPNT was added to chitosan solution as per the drug: polymer ratio. To this solution, TPP solution (1 mg/ml) was added slowly in stoichiometric amounts and solution was stirred well with magnetic stirrer. The nanoparticle solution thus formed was sonicated at 50% amplitude for 10 minutes. |
| C. Determination of particle size and zeta potential |
| Nanoparticles size and zeta potential was determined by dynamic light scattering method using Zetasizernano ZS (Malvern Instrument, UK). Six consecutive measurements were performed and average value of particle size and zeta potential was reported. Particle size was also determined by SEM (Carl Zeiss EVO-18, Germany). |
| D. Determination of encapsulation efficiency |
| The encapsulation efficiency of drug was found using UV-Visible spectrophotometer (UV-1800, Shimadzu, Japan). The drug showed maximum absorbance at 642 nm. A calibration chart was prepared for CPNT at 642 nm. Theoretical drug content was measured before nanoparticles formation. The chitosan nanoparticles were formed and centrifuged at 22000 rpm for 20 minutes at 4Ãâ¹ÃÅ¡C. Supernatant was collected and its free drug content was analysed. The encapsulation efficiency of nanoparticles was calculated as follows, |
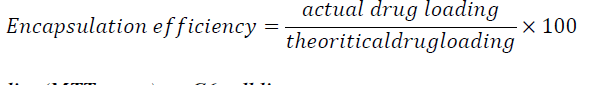 |
| E. Biological studies (MTT assay) on C6 cell line |
| Cytotoxic effect of the drug was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay [10, 11].The cell line used for this particular study was C6 (rat glioblastoma). Cells were maintained by using DMEM supplemented with 10% FBS and 1 X penicillin/streptomycin at 37Ãâ¹ÃÅ¡C in CO2 incubator (NUAIRE, DHD Auto flow automatic CO2 incubator; NU-5501/E/G.) in an atmosphere of humidified 5% CO2 and 95% air. The cells were maintained by routine sub culturing in 25 cm2 tissue culture flasks. Exponentially growing C6 cell lines were harvested from 25 cm2 Tissue culture flasks and a stock cell suspension (6 · 104) was prepared with media. 96-well flat bottom tissue culture plate was seeded with 100 μl of suitable media supplemented with 10% serum and allowed to attach for 24 hours. Cells grown in culture media alone and with appropriate concentration of DMSO were used as control and vehicle control, respectively. Test compounds were prepared just prior to the experiment and serially diluted with suitable medium to get the different concentrations Doxorubicin was used as standard drug with different concentrations. After 24 hrs of incubation, Cells were treated with 100 μl of test compounds from respective top stocks and the plates were again incubated for 48 hours. To each well of the 96 well plate, 20 μl of MTT reagent (Stock: 1mg/ml in PBS) was added and incubated for 4 hours at 37Ãâ¹ÃÅ¡C. Each treatment was performed in triplicates. The optical density (O.D) was measured by an ELISA reader at 570 nm. IC50 values of the compounds were obtained using the graph pad prism 5 software. |
III. RESULTS AND DISCUSSION |
| Following are the characterization data of CPNT. IR (KBr) [cm-1]; 1620(C=N str.),3090(Ar–H str.), 1495, 1380 (Ar.C=C str.), 820 (Ar.C–H def.), 750, 820, 840(naphthalene C–H), 1050, 1280 (Ar.C–O–C str.), 1H NMR (deuterated DMSO) [ppm]; 5.5 (2H,O–CH2), 6.9 (1H, pyrazole C–H), 7.1 (1H, pyrazole N–H),7.5-8 (11Ar–H), 13C–NMR : 174.1, 167.1, 162.4, 157.2,145.2, 136.1, 131.1, 129.4, 110.0, 105.9, MS(m/z); 458(M+),elemental analysis calcd.(%) for C23H15N6OClS: C, 60.19; H, 3.27; N, 18.32. Found(%): C, 60.06; H, 3.23; N, 18.34. |
| CPNT being water insoluble was encapsulated with chitosan nanoparticles to enhance its bioavailability. Initial experiments were carried out to investigate effect of chitosan concentration and CPNT:Chitosan ratio on particle size, zeta potential and encapsulation efficiency. Table 1 compares average particle size and zeta potential for various drug: polymer ratios. |
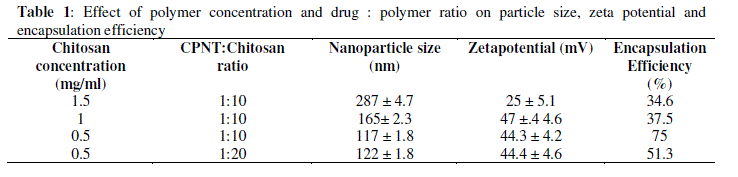 |
| As table indicates, high concentrations of chitosan lead to large particle size, with maximum particle size observed being 287 nm at 1.5 mg/ml of chitosan concentration with 32.5% encapsulation efficiency and zeta potential of 25.4 mV.Figure 2 demonstrates the absorbance characteristics of CPNT which were used to measure encapsulation efficiency. |
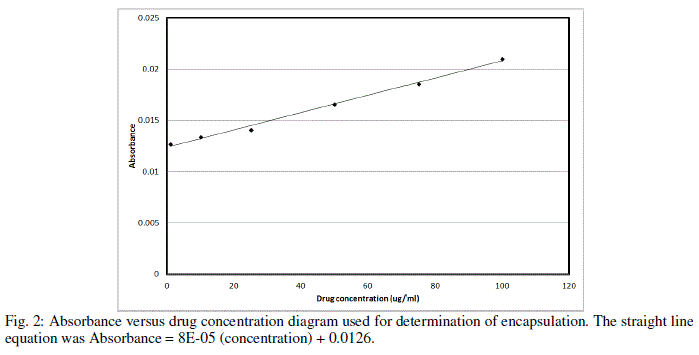 |
| Encapsulation efficiency increased and particle size reduced by decreasing chitosan concentration and best chitosan concentration was found to be 0.5 mg/ml. Drug encapsulation or loading efficiency was almost two folds when CPNT:Chitosan ratio of 1:10 was employed as compared to 1:20. The percentage drug encapsulation was 37.5 and 75%, respectively for CPNT:Chitosan ratio of 1:20 and 1:10, respectively. Availability of drug was more per mg of chitosan in solutions having less CPNT:Chitosan ratio which could have lead to higher encapsulation efficiency. Similar results were obtained by several authors [7, 8] while using various drugs and polymers. Finally, the formulation system was selected which produced stablenanoparticles with minimum size and highest drug encapsulation efficiency, i.e. chitosan concentration of 0.5 mg/ml and CPNT:Chitosan ratio of 1:10.SEM image, particle size curve and zeta potential for the optimized batch are shown in figures3 and 4, respectively. |
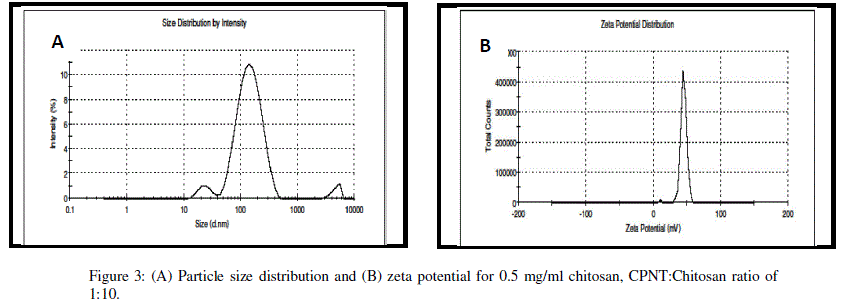 |
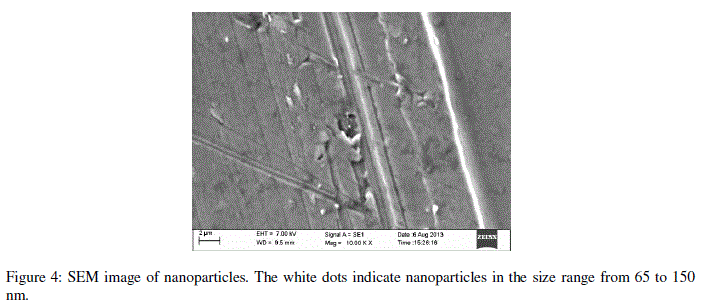 |
 |
| The biological activity of CPNT and chitosan encapsulated CPNT was compared on C6 cell lines and results are compared in Table 2. |
 |
| The IC50 value of chitosan encapsulatedCPNT and without encapsulation were 24 and 9 μg/ml, respectively. However, it should be noted that with 75% encapsulation efficiency of drug in 0.5 mg/ml chitosan solution, the loading capacity was found to be 0.075 μg/mg chitosan. Therefore out of 24 μg/ml of chitosan encapsulated drug, the amount of CPNT was 1.8 μg/ml, which is 5 times less than without encapsulation. Chitosan has recognized mucoadhesivity and ability to enhance the penetration of large molecules across mucosal surface [9] which increased bioavailability of CPNT and hence reduced IC50 value. |
IV. CONCLUSION |
| This study demonstrated synthesis of a novel, sparingly water soluble anticancer drug CPNT and compared its IC50 value with and without chitosan encapsulation at nano size. The CPNT was encapsulated in chitosan nanoparticles for different ratios of drug to chitosan. A very stable nanoparticle suspension of size 117 nm was obtained for drug to polymer ratio of 1:10 and polymer concentration of 0.5 mg/ml. Bioavailability of CPNT increased remarkably in conjugation with chitosan nanoparticles which was readily observed in IC50 value which was five times lower for chitosan encapsulated CPNT nanoparticles as compared to non-encapsulated CPNT and was 24 times lower than the standard Doxorubicin drug. |
References |
|