ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Amit Bharal*1, Vipan Kumar Sohpal 2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Lactobacillus acidophilus strain is one of the stable and acid-resistant, which can use for preserving food and antimicrobial activity against some human pathogenic microbes. In this paper, the ability of Lactobacillus acidophilus strains isolated from human feces studied. The bacteriocin produced strains grown in (pH 5.5, 370C for the 18-20 hour) MRS broth. The lactobacilli antibiotic sensitivity test performed against human pathogenic and food born microorganisms. The lactobacillus acidophilus of probiotic exhibited highly effective against P.aeruginosa and S.aureus culture with minimum inhibitory concentration (MIC) rate 40μl in 100 μl of MRS broth. From the result, it is concluded that bacteriocin can be added to foods as bio preservative and can also be used to treat the acute childhood diarrhea, and regulating immune response.
Keywords |
| Anti microbial activity, MIC, Bacteriocin, Probiotic, L.acidophilus, Antibiotic. |
INTRODUCTION |
| Probiotics are live health-promoting microorganisms that are incorporated into various kinds of foods. The ability of probiotics to withstand the normal acidic conditions of the gastric juices and the bactericidal activity of the bile salts, as well as the production of lactic acid that inhibits the growth of other microorganisms. It allows them to be established in the intestinal tract. Our objective was to isolate acid-resistant and bile-resistant variants of Lactobacillus acidophilus and to determine the antibacterial properties. An acidocin, a bacteriocin offspring of Lactobacillus acidophilus TK9201, is active against closely related lactic acid bacteria and food-borne pathogens including Listeria monocytogenes (Kanatani et al., 1995). Additionally, many of the organisms are not characterized for their acid shock response or the acid-tolerance response, which are known to vary among bacterial species (LAN-SZU CHOU and BART WEIMER 1999). This genus of microbe is broadly distributed in the environment. Several species, including lactobacillus acidophilus, are family members of the normal flora intestinal flora and vaginal flora of healthy humans being. Other species, such as Lactobacillus species, are commonly isolated from dairy products as well as from fruits and vegetables. They play a significant role as probiotics in human diets and animal nutrition(Amin,Farzane,shafiee,.2012). Several strains of Lactobacillus delbrueckii ssp. Bulgaricus and Streptococcus thermophilus, which produced exocellular polysaccharides (EPS), varied in the amount produced and they report indicating the presence of bacteriocin-like activity in animal manure compost (Pigeon et al., 2002 and Abdel et al. 2010). Lactic acid bacteria, including Lactobacillus species, which have been used for preserving food by fermentation method for many years. It can serve a dual action by acting as agent for food fermentation and, in additionally, potentially provides health benefits. Bacteriocins are proteinaceous toxins produced by bacteria, which inhibit the growth of similar or closely related bacterial strain. They are typically considered narrow spectrum antibiotics (Farkas-Himsley HANNAH, H 1980). Lactic acid bacteria is useful as a health adjunct and are commonly added to food as the delivery mechanism (Chou et al., 1999). Biodiversity analysis and accurately monitor changes in response to the diet, which help determine the role of probiotic, prebiotics and symbiotic in health promotion (Nagpal et al., 2007). Several LAB bacteriocins offer potential applications in food preservation, and the use of bacteriocins in the food industry and current findings supporting an interaction between extracellular proteins/peptides produced by probiotic bacteria (strains (Gálvez et al., 2007,Sánchez et al., 2010) |
| The intention of this study was to investigate the antibacterial activity of lactobacillus strains of vaginal origin. Furthermore, we sought to characterize bacteriocins for their structural properties and determine their antimicrobial activities against some common human pathogens including, P.aeruginosa, B.subtilis, E.coli, S.pyogenes, S.aureus, M.luteus, S.typhi and S.faecalis |
MATERIAL AND METHODOLOGY |
| Preparation of Bacteriocin |
| The bacteriocin-producing strains were grown in MRS broth (pH 5.5) at 37 C for 18-20 h. The lactobacilli culture was centrifuged at 8000-9000 rpm for 5-6 min, and then the supernatant was adjusted to pH 6.5-7.0 with 1N NaOH and stored at favourable conditions for further usage. Bacteriocin activity was detected by the agar-spot test. The test was performed as follows: 200 micro liter of each Lactobacillus culture at the early exponential growth phase (OD at 600nm of 0.2-0.3) in MRS broth was mixed with 4 ml of MRS soft agar (0.6% agar, pre warmed to 480C) and poured on an MRS agar plate. Then, 3 micro liter of each culture supernatant was dropped onto the solidified soft agar. The plates were incubated for 48 h in a candle jar. Inhibition of bacteriocin was indicated by a zone on the soft agar layer. |
| Test culture Preparation |
| The pathogenic microbial strains namely Pseudomonas.aeruginosa (MTCC4676), Staphylococcus.albus (MTCC7407), Staphylococcus.aureus (MTCC7405), Bacillus.subtilis(MTCC3053), Escherichia.coli (MTCC1674) and Streptococcus. pyogenes(MTCC 1926), Micrococcus.luteus (MTCC4428) obtained from Institute of Microbial Technology, Chandigarh and used for evaluating the antibacterial activity. Theses cultures are to be revived in a healthy state. At least three to four colonies of the same physiology were selected from an agar plate culture. The growth of the culture in the form of those colonies were picked up and transferred into a suitable broth medium of 4-5ml tube. The broth culture was incubated at 35C (usually 2 to 6 hours). |
| Inoculation of Test Plates |
| Muller Hilton agar media was used for antimicrobial activity analysis. After the incubation of the broth cultures, a sterile cotton swab was dipped into the suspension. The swab was rotated several times and pressed firmly on the inside wall of the tube above the fluid level, thus removing the excess inoculum from the swab. The dried surface of a Muller-Hinton agar plate was inoculated by spreading or streaking the swab on the entire agar surface. This procedure was repeated two more times, rotating the plate approximately at an angle 600 each time to ensure an even distribution of inoculum. |
| Agar well diffusion assay |
| Pouring bactericin onto Inoculated Agar Plates |
| The wells were then cut with 6mm diameter using a cork borer and about 30 micro liters of the crude bacteriocin were poured into each well. Not more than six wells were cut into each plate. The plates were inverted and placed in an incubator set to 350C within 15 minutes after the pouring of bacteriocin. |
| Reading Plates and Interpreting Results |
| After 16 to 18 hours of incubation, each plate was examined. The diameters of the zones of complete inhibition (as judged by the unaided eye) were measured, including the diameter of the well. Zones were measured to the nearest whole millimeter, using sliding calipers or a ruler, which was held on the back of the inverted Petri plate. The Petri plate was held a few inches above a black, nonreflecting background and illuminated with reflected light. The observations were then kept as a photographic record. |
RESULTS |
| Antimicrobial Assay observations |
| In this susceptibility test, the concentration of the extract used was 40μl/100μl and bacteriocin is to be stored in MRS media. This sensitivity test yielded the zones of inhibition of varying sizes (recorded by measuring the diameter of the zone). The MRS media referred to as the negative control. |
| Case-I: Antimicrobial Assay of the Bacteriocin (B. acidophilus) against S.typhii and Micrococcus:. The antibiotic Meropenam used as a positive control on S.typhii and Imipenam on the Micrococcus luteus and size of inhibiting zone is shown in table 1. |
| Table1: Antimicrobial Assay of the Bacteriocin (B. acidophilus) against S.typhii and M. luteus, S.faecalis and B. subtillis S.aureous, P. aeruginosa, S.albus, S.pyogenes and E.coli |
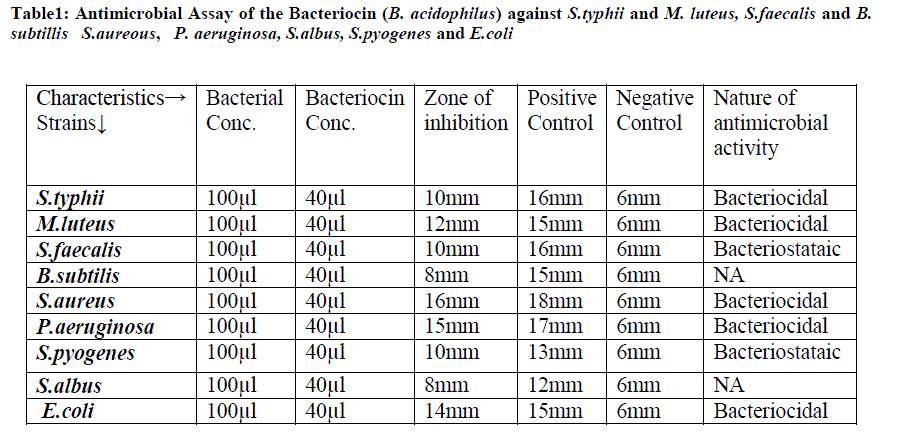 |
| Case-II: Antimicrobial Assay of the bacteriocin (B. acidophilus) was analyzed against S.faecalis and B.subtilis. The cholin antibiotic was used as positive control on S.faecalis and Doripenam on the B.subtilis and size of inhibiting zone is shown in Table.1 as results of bacteriocin as shown in figure1. |
| Case-III: Antimicrobial Assay of the bacteriocin (B. acidophilus) was analyzed against S.aureus and P.aeruginosa. The antibiotic Colistin was used as a positive control on S.aureus and Doripenam on the P.aeruginosa . and size of inhibiting zone is shown in table.1 |
| Case-IV: Antimicrobial Assay of the Bacteriocin (B. acidophilus) was analyzed against S.albus, S.pyogenes and E.coli. The antibiotic Colistin was used as a positive control on S.albus and Doripenam on the S.pyogenes and E.coli and size of inhibiting zone is shown in table.1 |
DISCUSSION |
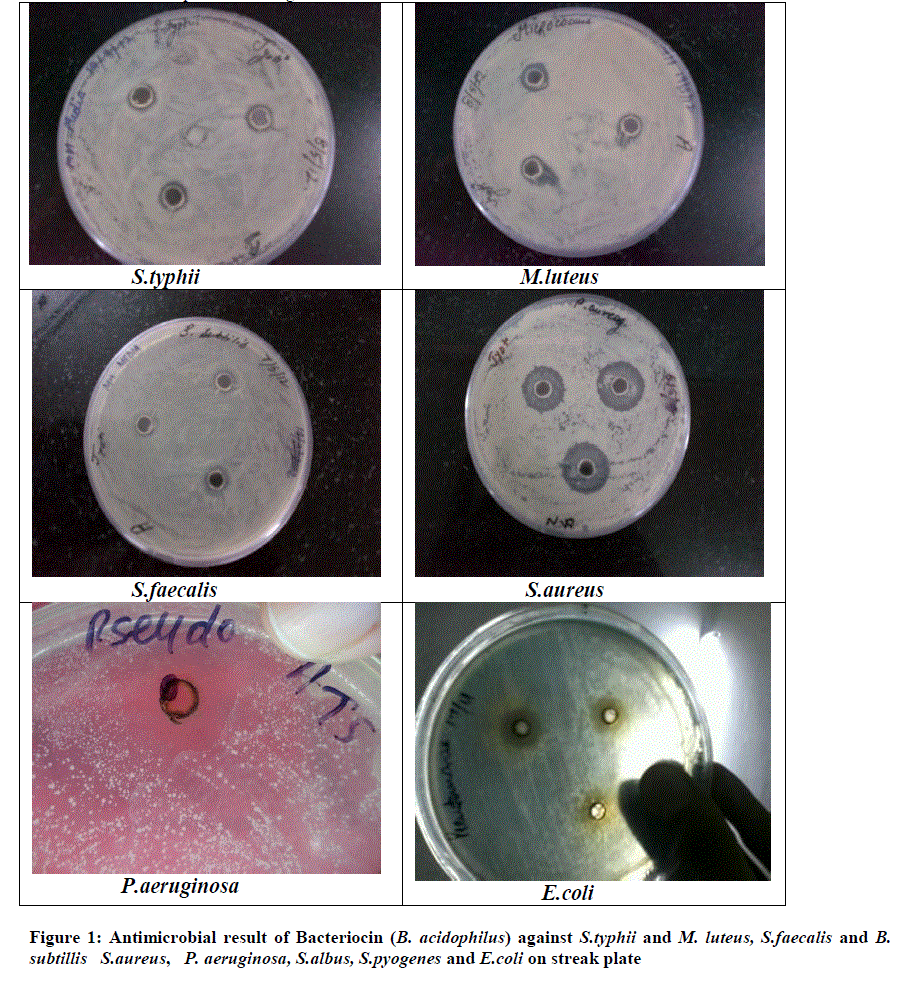
| A Lactic acid bacteria can produce antagonistic compounds that vary in their spectra of activity. The antimicrobial agent from strain L. acidophilus demonstrated a wide range and strong antimicrobial activity against both gram-positive and gram-negative bacteria. An acidocin, an antimicrobial peptide produced by L .acidophilus showed inhibitory activity against major food pathogens and spoilage bacteria. This bacteriocin, which inhibits several gram-positive and gram-negative bacteria activity, seems to be most active against S.aureus and P.aeruginosa. it was evaluated the possibility of antimicrobial substances against oral microbial plaque. According to the study of Amin and result, found that the L. plantarum can produce antimicrobial compounds and these bacteria exist in fresh vegetables; consume of such vegetables may colonize this probiotic and other useful probiotic in the mouth and intestines and protect these parts of the body from pathogens (Amin, 2011).. However, the effect may depend on the specific assay conditions, such as the amount and purity of the bacteriocin. In both cases, inhibition is most likely due to weakening of the outer plasma membrane following sequestration of magnesium ions by the chelating. Antimicrobial activity has been associated with molecules frequently exported by bacteria, such as hemolysins or hydrolytic enzymes. Bacteriocin plantaricin KW30 were also susceptible to digestion by various proteases. Furthermore, the antimicrobial agent demonstrated mode of action as the instantaneously reduce in the optical density of P. aeruginosa indicated cell lysis. L. acidophilus La-14 to produces bacteriocin active against L. monocytogenes Scott A (1600 AU/ml) in MRS broth at 30°Cor 37°C. Their work concluded that L. acidophilus La-14 shows a good resistance to several drugs and may be applied in combination for therapeutic use (Todorov et al., 2011). The viable lactobacilli can inhibit food-borne and enteric pathogenic microorganisms and other antimicrobial substances, like yogurt and acidophilus milk have been considered to be healthy probiotic diets. Bacteriocin characterized in this study found that antibacterial activity at a pH range of 3.0 to 5.0. In addition, L. acidophilus strain occurred between pH 3.0 and 5.0 and the inhibitory activity was lost when the pH was raised to 5.3. The activity and zone formation are represented in figure 1 |
 |
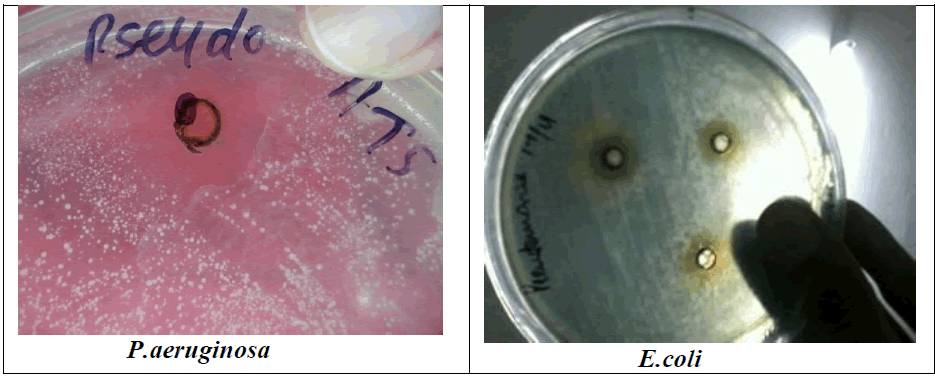 |
| Figure 1: Antimicrobial result of Bacteriocin (B. acidophilus) against S.typhii and M. luteus, S.faecalis and B. subtillis S.aureus, P. aeruginosa, S.albus, S.pyogenes and E.coli on streak plate |
CONCLUSION |
| The lactobacillus acidophilus of probiotic found to be active against Pseudomonas aeruginosa and staphylococcus aureus with minimum inhibitory concentration (MIC) rate 40μl in 100 μl of MRS broth. The lactobacillus acidophilus also exhibited antimicrobial activity against many food born harmful bacteria that cause various diseases. So thus we can say that bacteriocin (lactobacillus acidophilus) having antimicrobial activity and signifies that bacteriocin can be added to foods in the form of concentrated preparations as food bio preservatives and can also be used to treat these harmful bacteria oriented acute diseases.. |
References |
|