ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Manoj Kumar Manugunta and Naveena Kanaboyana Assistant Professors, Department of Civil Engineering, Annamacharya Institute of Technology and Sciences(AITS), ,Rajampet, Andra pradesh India |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Everyday Portland cement can be a critical product used in generation involving concrete floor. Even though manufacturing of OPC involves many disadvantages such as depletion involving garbage just like lime green natural stone plus the clay-based. Every single great deal of bare cement emits identical quantity of CARBON DIOXIDE. One alternative is actually Geopolymer that is an eco-friendly substance and sensible energy gain. The main substance in addition to structural characteristics linked to geopolymer resulting from travel lung burning ash in addition to slag are generally identified intended for the aftermaths linked to organic substance collection for your houses linked to geopolymer composites. Every one of the materials applied had been indicated with regard to real, chemical substance, morphological in addition to mineralogical qualities. The particular placing qualities of the geopolymer composite had been decided. Geopolymer mortar cubes are generally throw employing fly lung burning ash, soil granulated crank air conditioner slag (GGBS) seeing that binders having alkaline alternative The particular accessible codal conventions had been implemented to be able to throw in addition to test out the specimens. The particular movement feature of the mortar was decided within fresh talk about. The particular specimens had been tested with regard to compressive strength at unique ages. The particular growth regarding compressive strength of the prevents was analyzed to have the perfect mix. Based on the results, the feasibility regarding employing geopolymer mortar prohibit being a structural system was figured out. These resources employed for the analysis have been located ideal for generating geopolymer mortar. The results in the analysis expose the maximum toughness formulated within the mortar for your combined 80% GGBS as well as 20% Journey lung burning ash. It was located 33.59 N/mm2 at the age of 1 week for your fluid-to-binder ratio associated with 0.45. This geopolymer mortar builds up toughness at normal circumstances with no regular healing. This compressive toughness in the geopolymer mortar does raise while using the GGBS content material pertaining to different fluid in order to binder percentages
Keywords |
| Geopolymer mortor, GGBS, CO2, flyash, 12 molarities |
INTRODUCTION |
| Geopolymer materials represent an innovative that is generating considerable interest in the construction industry, particularly in light of the ongoing emphasis on sustainability. In contrast to Portland cement, most Geopolymer systems rely on minimally processed natural materials or industrial by-products to provide the binding agents. Since Portland cement is responsible for upward of 85 percent of the energy and 90 percent of the carbon dioxide attributed to a typical ready-mixed concrete, the potential energy and carbon dioxide savings through the use of Geopolymers can be considerable. Consequently, there is growing interest in Geopolymer applications in transportation infrastructure. Unlike ordinary Portland/pozzolanic cements, geopolymers do not form calcium- silicate-hydrates (CSHs) for matrix formation and strength, but utilize the poly condensation of silica and alumina precursors to attain structural strength. Two main constituents of geopolymers are: source materials and alkaline liquids. The source materials on alumino-silicate should be rich in silicon (Si) and aluminium (Al).They could be by-product materials such as fly ash, silica fume, slag, rice-husk ash, red soil, etc. |
Unlike ordinary Portland/pozzolanic cements, geopolymers do not form calcium- silicate-hydrates (CSHs) for matrix formation and strength, but utilise the poly condensation of silica and alumina precursors to attain structural strength. Two main constituents of geopolymers are: source materials and alkaline liquids. The source materials on alumino-silicate should be rich in silicon (Si) and aluminium (Al).They could be by-product materials such as fly ash, silica fume, slag, rice-husk ash, redmud, etc. Comparison of conventional and geopolymer cement is shown in Fig. 1.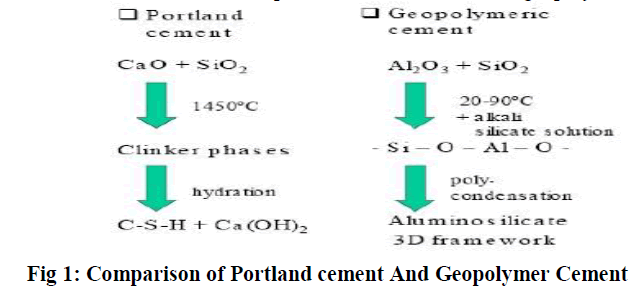 |
| Conceptually, the formation of geopolymers is quite simple. In the case of geopolymers based on aluminosilicate, suitable source materials must be rich in amorphous forms of Si and Al, including those processed from natural mineral and clay deposits (e.g., kaolinite clays) or industrial by products (e.g., low calcium oxide ASTM C618 Class F fly ash Or ground granulated blast furnace slag) or combinations thereof. In the case of geopolymers made from fly ash, the role of calcium in these systems is very important, because its presence can result in flash setting and therefore must be carefully controlled (Lloyd and Rangan 2009). The source material is mixed with an activating solution that provides the alkalinity (sodium hydroxide or potassium hydroxide are often used) needed to liberate the Si and Al and possibly with an additional source of silica (sodium silicate is most commonly used). |
| Geopolymers are mineral polymers from the geochemistry process. Geopolymer starting materials have to consist of specified amount of silica and alumina which is dissolved in alkaline medium. Consequently stable polymeric networks of alumosilicates are forming. Geopolymeric materials possess good mechanical properties, including fire and acid resistance. Mentioned properties make geopolymers as alternative construction material. It is reasonable to emphasize that the type and nature of the used starting material will directly affect the final physical and chemical properties of Geopolymer. Kaolinite is the most often used raw material because of its high content of alumina .It is thermally activated to transform into amorphous metakaolinite which is more soluble in basic solution. Searching for another low cost material is leading to illite clays – materials. |
RELATED WORK |
CHARACTERIZATION OF MATERIALS |
| Material that contains mostly Silicon (Si) and Aluminium (Al) in amorphous form are all possible source materials for the manufacture of geopolymer. Manufacture of GEOPOLYMER by Several minerals and industrial byproduct materials have been investigated in the past by many researchers. |
| Ground-granulated blast-furnace slag (GGBS or GGBFS) is obtained by quenching molten iron slag (a byproduct of iron and steel-making) from a blast furnace in water or steam, to produce a glassy, granular product that is then dried and ground into a fine powder |
Characteristics of GGBS |
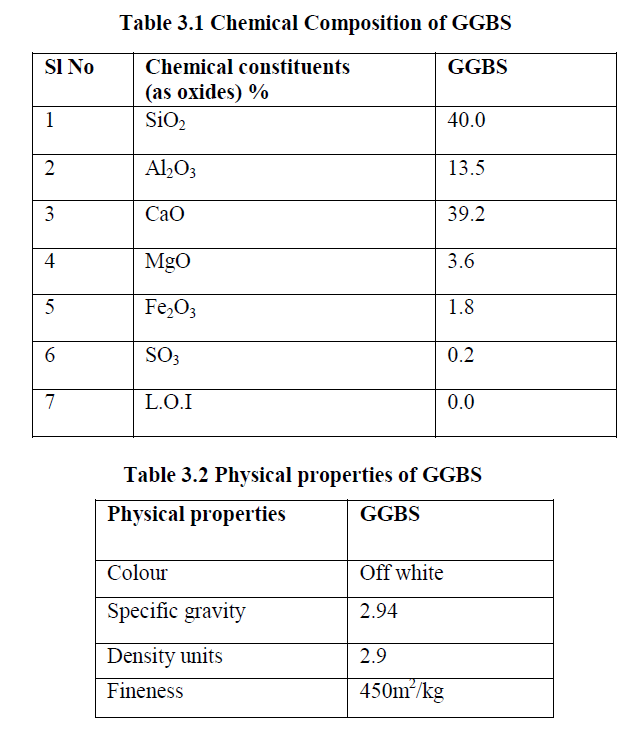
| The chemical composition and physical characteristics of GGBS were determined and the results are tabulated in Tables 3.1.and 3.2 |
 |
Fly Ash |
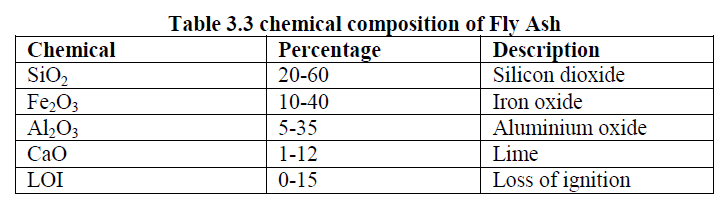
| Flyash is one of the residues generated in the combustion of coal. Fly ash is generally captured from the chimneys of coal tired power plants and is one of the two types of ash that jointly are known as coal ash the other bottom ash is removed from the bottom of coal furnaces. Depending upon the source and make up of the coal being burned, the components of fly ash vary considerably but all fly ash includes various chemical composition which was shown in Table 3.3 having substantial amounts of silicondioxide (SiO2) and calcium oxide (Cao). Toxic constituents include cobalt, lead, manganese, mercury, arsenic, beryllium, boron, cadmium, chromium, Malybdenum, selenium, etc. |
Characteristics of Fly Ash |
 |
| The size of fly ash particles range from 0.5μmm to 100. μmm. Two classes of fly ash are defined by ASTMC 618 class F and class C fly ash. The chief difference between these classes is the amount of calcium, silica, alumina and iron content in the ash. |
Characteristics of Fine Aggregate |
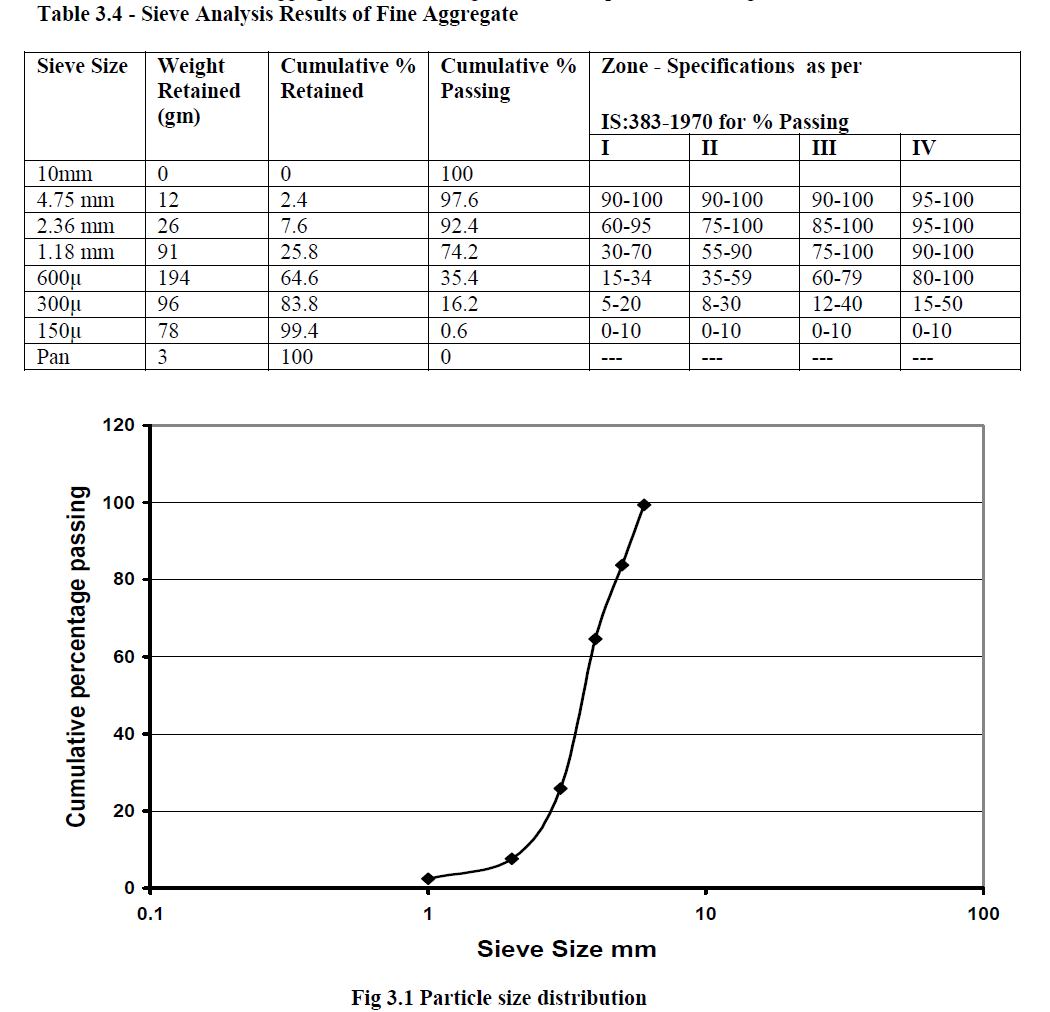
| The particle size distributions of sand were determined by plotting as shown in Fig 3.1 and the results are tabulated in Table 3.4. The sand (Fine aggregate) is confirming to zone III as per IS 383having fineness modulus as 2.83. |
 |
Alkaline Solution |
| The Alkaline solution used for experimental investigation is a combination of Sodium silicate solution and Sodium Hydroxide solution. It is seen that the Geopolymers with Sodium Hydroxide solution exhibit better Zeolitic properties than Potassium Hydroxide activated Geopolymers. Also it has been confirmed that addition of Sodium Silicate Solution to Sodium Hydroxide enhanced the reaction rate between Source material and the alkaline solution. A combination of sodium silicate solution and sodium hydroxide solution was chosen as the alkaline liquid. Sodiumbased solutions were chosen because they were cheaper than Potassium-based solutions. |
Sodium Hydroxide |
| The Sodium Hydroxide is in flakes and pellet form with about 98% purity. These pellets were mixed with distilled water to obtain the sodium hydroxide solution of required molarity. In the present study, The Molarity of the solution is kept constant at 12M for all the experimental investigations. NaOH is also commonly used as an alkaline activator in geopolymer production. While it does not maintain the level of activation as a K+ ion, sodium cations are smaller than potassium cations and can migrate throughout the paste network with much less effort promoting better zeolitization. Furthermore, it bears a high charge density which promotes additional zeolitic formation energy.The concentration and molarity of this activating solution determines the resulting paste properties. While high NaOH additions accelerate chemical dissolution, it depresses ettringite and CH (carbon-hydrogen) formation during binder formation. Furthermore, higher concentrations of NaOH promote higher strengths at early stages of reaction, but the strength of aged materials were compromised due to excessive OH- in solution causing undesirable morphology and non-uniformity of the final products. It is found that geopolymers activated with sodium hydroxide develop greater crystallinity thus improving stability in aggressive environments of sulfates and acids. Additionally, the use of sodium hydroxide as an activator buffers the pH of pore fluids, regulates hydration activity and directly affects the formation of the main C-S-H product in geopolymer pastes. |
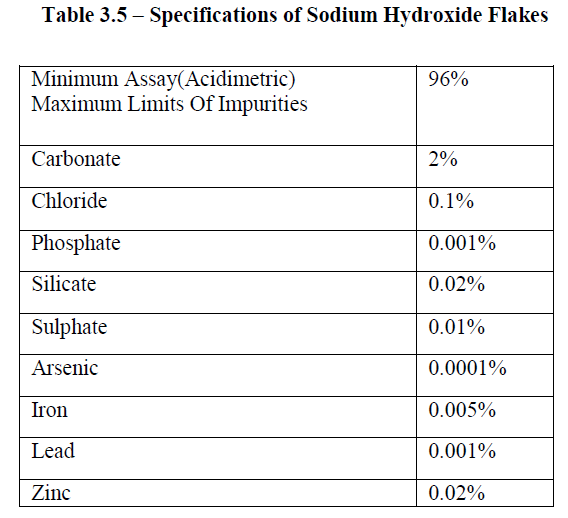
| The sodium hydroxide solids were either a technical grade in flakes form, with a molecular weight of 40 and obtained from Dutta scientific chemicals was used. The specifications of sodium hydroxide flakes is as shown in Table 3.5 |
 |
Sodium Meta Silicate (Na2SiO39H2O) |
| Sodium (or potassium) silicates are manufactured by fusing sand (SiO2) with sodium or potassium carbonate (Na2CO3 or K2CO3) at temperatures in excess of 1100 °C and dissolving the product with high pressure steam into a semi-viscous liquid referred to as waterglass. Waterglass is rarely used as an independent activating unit, because it does not possess enough activation potential to initiate pozzolanic reaction alone. Rather, it is commonly mixed with NaOH or KOH as a fortifying agent to enhance alkalinity and increase overall specimen strength. The most common alkaline liquid used in geopolymerization is a combination of sodium hydroxide or potassium hydroxide and sodium silicate or potassium silicate . Sodium silicate solution is commercially available in different grades, but it should be noted that powdered waterglass leads to lower performance compared to the liquid form. For best results, a silicate solution with a SiO2 to Na2O ratio (by mass) of 2.0 mixed with an 8–16 M activator 24 hours prior to use is recommended . |
| The most important property of this product is its mass ratio of SiO2 to Na2O, which is commercially available in the range of 1.5 to 3.2 (with 3.2 being the best suited for geopolymerization). Soluble silicates reduce alkali saturation in pore solution and promote greater interparticle bonding with both geopolymer binders and the included aggregate material . Testing has revealed that activating solutions containing little or no soluble silicates produced significantly weaker compressive strengths of mortars and concretes than those activated with high doses of soluble silicates. As well, the presence of such silicate material improves interfacial bonding between rock aggregates and geopolymer mortars. On the contrary, additional research shows that under increasing temperatures, specimens containing waterglass decrease in strength while those containing only a base activator (NaOH, KOH) produce higher strengths. Additional research is still required to accurately determine the specific effects produced through the addition of water glass into geopolymer binder solutions. Sodium silicate powder is obtained from laboratory reagents and fine chemical, from dutta scientific chemicals was used. |
| Sometimes an image may contain text embedded on to it. Detecting and recognizing these characters can be very important, and removing these is important in the context of removing indirect advertisements, and for aesthetic reasons. |
| Our system aims at the automatic detection of text. This is done by the algorithm. Fig. 1 shows the flow diagram of text detection algorithm. The algorithm steps are summarized as follows. |
| 1. An efficient edge detection scheme is applied to the greyscale image. The image I is blurred (to reduce false edges and over-segmentation) using open-close and close-open filters. The final blurred image Ib is the average of the outputs of these filters. The 3 x 3 8-connected structuring element of type „squareâÃâ¬ÃŸ is used here. Next, the morphological gradient operator is applied to the blurred image Ib resulting in an image G as follows: G = Dilation (Ib) – Erosion (Ib) |
| The Morphological gradient is an edge-strength extraction operator that gives symmetric edges between foreground and background regions. |
| The resulting image is then thresholded to obtain a binary edge image. Global thresholding technique is used for that. |
| 2. Closed edges in the binary edge image are grouped by dilation using eight- connected structuring elements. Then small connected components in the dilated image are filtered using erosion. The output is a binary image that contains text candidate regions. |
| 3. Connected component labelling is performed to label each object separately. |
| 4. After applying connected component labelling, the first set of criteria is applied which eliminate all objects whose area is greater than 10000 and filled area is greater than 8000. One more criteria namely major axis length is used which is used to retain the text region alone. All objects, whose major axis lengths are in between 20 to 3000, are considered to be text. To eliminate small objects, connected component labelling is applied to the resultant image and the second set of criteria is applied which eliminates all the objects whose area is less than 300 and filled area is less than 500. |
| After applying all these 4 steps, we get a filtered image that contains only text regions. |
EXPERIMENTAL RESULTS |
| The material used of in the presence research is as follows: |
| 1. GGBS (Ground Granulated Blast Furnace Slag) |
| 2. Fly ash |
| 3. Sand |
| 4. Alkaline Solution |
Storage |
| The materials were procured from respective places as mentioned above. Further the task ahead was the storage of these materials. The materials had to be stored in dry place, which is free from moisture, as these materials have tendency to deteriorate and lose their properties. Therefore extra care should be taken in this regard. The materials were stored in the laboratory. Hence, sufficient care was needed to keep these materials intact without any wastage at the same time attaining the optimum usage of the materials. |
Preparation of Alkaline Solution |
| Distilled water was used to prepare alkaline solution to avoid any mineral interference. The alkali solution has to be prepared 24 hours advance before use. The sodium hydroxide is available in small flakes and sodium silicate is available in crystal or gel form depending on the required solution of different molarity has to be prepared. |
| The mass of NaOH solids in a solution varied depending on the concentration of the solution expressed in terms of molar, M. For instance, NaOH solution with a concentration of 12M consisted of 12x40 = 480 grams of NaOH solids (in flake or pellet form) per litre of the solution, where 40 is the molecular weight of NaOH. Note that the mass of NaOH solids was only a fraction of the mass of the NaOH solution, and water is the major component. The sodium silicate is taken double of sodium hydroxide for preparing the solution. |
| The solution normally soapy in nature and even a drop of solution falls on the skin, it may cause skin irritation etc.., hence proper care and precautions should be taken while handling the solution. The solution was stored in closed containers with proper label |
Preparation of Geopolymer mortar Samples |
| 1. A Geopolymer motor cube was prepared by using Fly ash and Ground granulated blast furnace slag (GGBFS) and locally available fine aggregate which is passing through 4.75 mm IS Sieve. |
| 2. The alkaline solution which includes Sodium Hydroxide and Sodium silicate which was mixed in water as per 12 Molarity basis by the ratio 1:2 for the different Fluid to Binder. |
| 3. The alkaline solution was prepared one day prior to do the Geopolymer mortar cubes. |
| 4. The experimental program was conducted on next day by weighing the materials like Fly ash, GGBFS and fine aggregate and mixed uniformly for 3 min at the ratio of 1:2. |
| 5. The samples of Geopolymer mortar was obtained by mixing the binder and fine aggregate with alkaline solution for another 3 to 5 min. |
| 6. The mortar was placed in 70.6mm x 70.6mm x 70.6mm mould in three equal layers and compacted well and then the samples were placed in vibrator for further compaction. |
| 7. Three cubes were prepared to obtain the compressive strength for the different ages i.e. 1 day, 3 days and 7 days respectively and the samples were subjected to ambient curing. |
| 8. At the next day, the Geopolymer mortar cubes were demoulded which was shown in Fig 4.1 and average compressive strength for one day was obtained by placing the samples in universal testing machine as shown in Fig 4.2 Repeating the same procedure for obtaining the average compressive strength for 3 days and 7 days respectively. |
| 9. After the testing the sample from the Universal Testing Machine (UTM), the cracking pattern of the Geopolymer mortar cubes is obtained as shown in Fig 4.3. The cracking pattern varies with the Fluid to Binder ratio and combinations of the binding material. |
| 10. For lower fluid to Binder ratio like 0.40, the samples are very dry due to lack of reaction of alkaline solution with binders. When these samples are subjected to compressive stress, the crushing pattern of a Geopolymer cubes are obtained like a powder form |
Compressive strength Development |
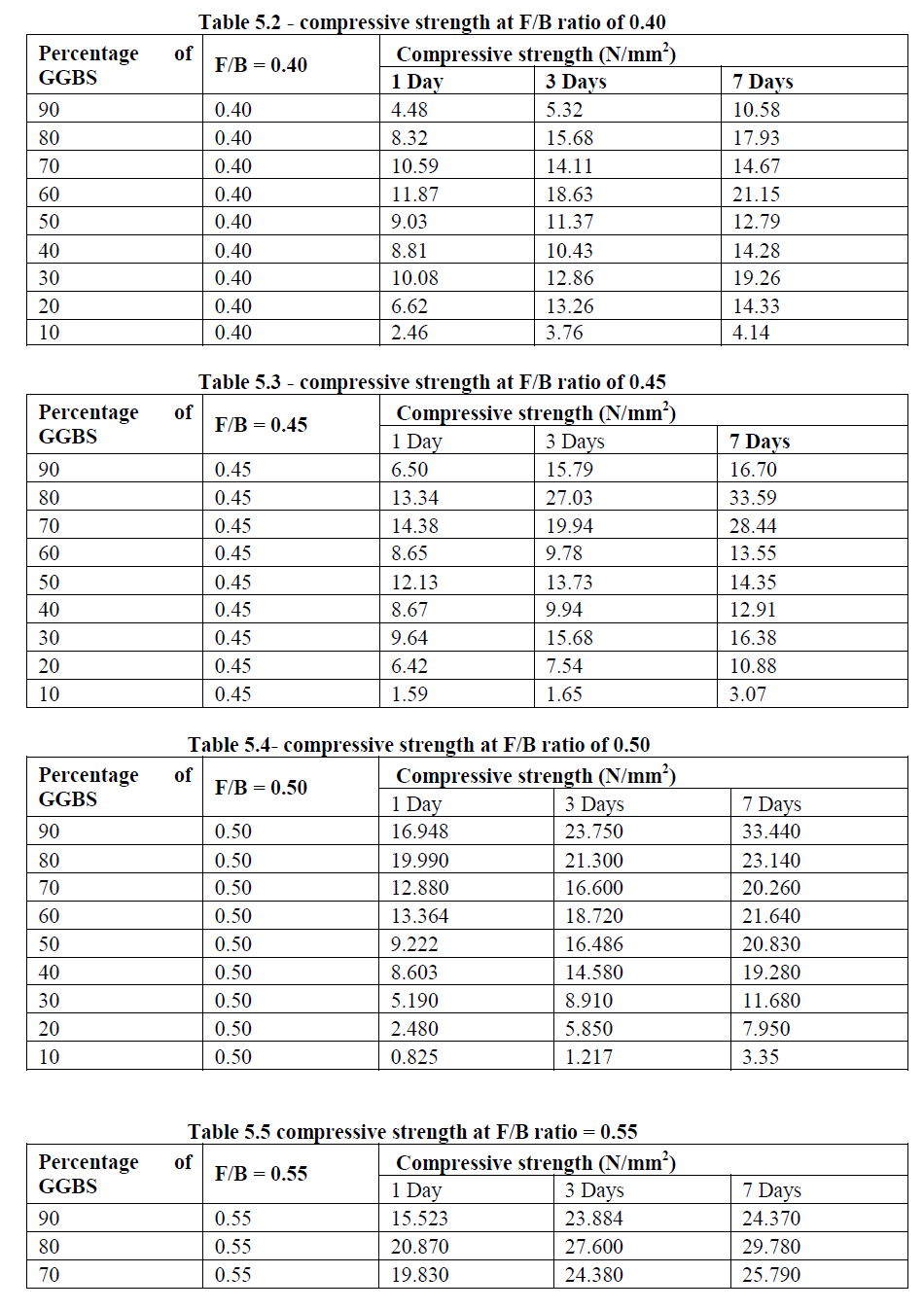
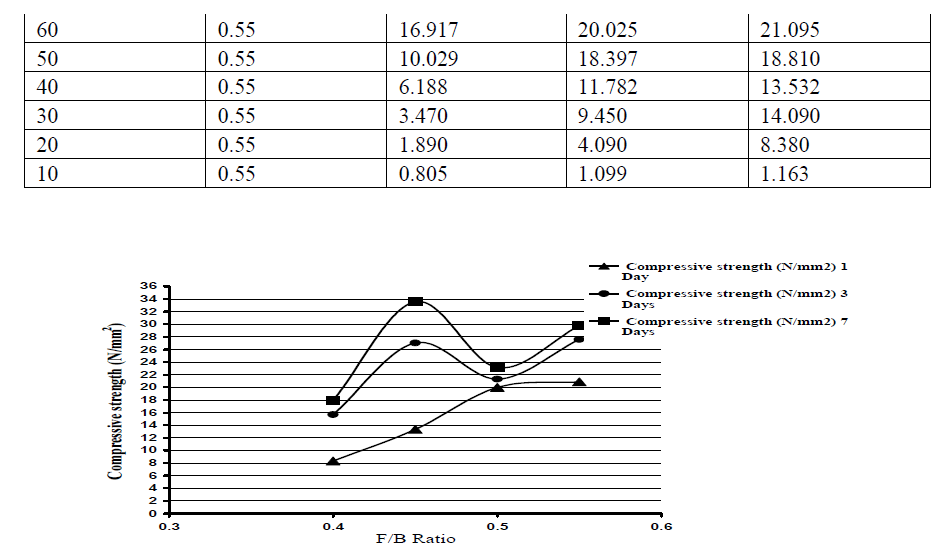
| The strength development of mortar cubes were found using two binders-fly ash and GGBS in different proportions. The quantity of fly Ash and GGBS were varied from 10% to 90% at an increment of 10%. In general, as the percentage of GGBS increases strength development was increased. But higher the GGBS content, there is no complete reaction between the GGBS content and the Fluid for 0.40 and 0.45 ratios respectively. From the Table 5.2, the maximum compressive strength of 21.15 N/mm2 was obtained at F/B ratio of 0.40 for 60% GGBS for 7 days. Similarly at F/B ratio of 0.45 and 0.50, the maximum compressive strength of 33.59 N/mm2 and 33.44 N/mm2was obtained at the age of 7 days for 80% GGBS as shown in Table 5.3 and 5.4 respectively. The Table 5.5 shows that there was an early strength development at the age of one day is 20.87 N/mm2 for the Fluid to binder ratio of 0.55 at a 80% GGBS and it increases with an age factor of 3 and 7 days as 27.60 N/mm2 and 29.79 N/mm2 respectively. The figs 5.2- 5.5 show compressive strength of different percentage of GGBS with different Fluid to Binder ratio. |
 |
 |
CONCLUSION |
| 1. The materials like Fly Ash and GGBS as binders with alkaline solution which includes Sodium Hydroxide flakes and Sodium Meta Silicate (commercial grade) are suitable to prepare Geopolymer mortar. |
| 2. The compressive strength is increased with increase in GGBS content. But the mix was dry for F/B ratio of 0.40 and 0.45 respectively. The compressive strength at the age of 7 days are in the range of 1.163- 33.59N/mm2 |
| 3. The flow is very dry and greater percentage of flow for F/B ratio of 0.40 and 0.45 respectively. |
| 4. At all the proportions, the compressive strength is increased with increase in age. The maximum strength is attained for F/B ratio of 0.45 at 7 days for the combination of 80% GGBS and 20% Fly Ash. |
| 5. The reaction between the Fly ash and alkaline solution gives low strength for higher F/B ratios like 0.50 and 0.55 respectively. But for the F/B ratio of 0.45, the compressive strength for F/B ratios of 0.40 and 0.45 respectively gives satisfactory results when compared to higher F/B ratios like 0.50 and 0.55 respectively. |
| 6. Locally available sand was used for preparation of Geopolymer mortar with binders and alkaline solution. By conducting fineness modulus of sand, we conclude that it was a medium sand having fineness modulus of 2.83 which is satisfactory for making mortar. |
References |
|