ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
A. Mandal1 and T.P. Sastry2*
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Recently, focus is directed on research which involves synthesis of nanoparticles using natural polymericmaterials that are eco friendly, inexpensive as well as biodegradable. In this study, bimetallic silver-gold nanoparticles (Ag-AuNPs) were synthesized usinggelatinextracted from chrome containing leather waste as a template.The nanoparticles were confirmed with UV-Vis and particle size analysis. The gelatin directed bimetallic nanoparticles were air driedto obtain as nanobiocomposites (NBCs). These NBCs were characterized via FTIR, DSC, TGA, XRDand SEM/EDX. Also, mechanical properties such as tensile strength and Young’s modulus along with water absorption studies of the NBCs were conducted.The results indicate that the gelatin NBCs incorporated with bimetallicnanoparticles are biodegradable and possess the desired mechanical propertiesneededfor biomedical applications. Moreover, the in vitro results demonstrate the viability of fibroblast (NIH 3T3) cells with gelatin based NBCs.The gelatin NBCincorporated with Ag-Au NPs isbiocompatible and can be used as scaffolds which have potential for various biomedical applications.
Keywords |
| Gelatin, Bimetallic, Silver, Gold, Nanoparticles, Template, Nanobiocomposites. |
INTRODUCTION |
| Gelatin, a derivative from the most abundant biopolymer collagen has been used in biomedical applications [1]. The physico-chemical and biological properties of gelatin render it as candidate suitable for fabricating composites and tissue engineered scaffolds [2-5]. Additionally, it is much cheaper compared to collagen and has reduced antigenicity which essentially makes it easily available and convenient to use as biomaterial [6-8]. It is well known that temperature above 37ºC results in gelatin polypeptide chains to unfold and functional groups such as –NH2, – SH and –COOH present in these chains can serve as a template for synthesis of nanoparticles and in nano-sized devices fabrication [9]. |
| Gold nanoparticles(AuNPs) possess unique optical properties and have found use in catalysis and in electronic devices [10]. Also, they have been used as therapeutic agents, sensory probes and in delivery of drugsfor biomedical applications [11,12].Similarly, silver nanoparticles also exhibit distinct optical activities that have found wide use in electronics, catalysis and in sensingbased applications [13,14]. Moreover, it displays antimicrobial activity against a broad spectrum of bacteria and fungi and thus finds use as a biocide and also in the preparation of bactericidal nanomaterials for wound dressings and surgical purposes [15-17]. |
| The high surface energy and van der waal forces present in both silver and gold nanoparticle solutionsresultin their aggregation and therefore, capping of these nanoparticles is essential to avoid this phenomena [11]. Hence, there is a need and thus research towards syntheses of NPs using natural polymers that are eco friendly, cost effective as well as renewable materials have drawn attention.The side chain amino acids of the polymer such as gelatin interactmore effectively with thesenanoparticle surfaces. Thehigh surface area of these nanoparticles enables more non specific interactions with the side chain amino acids of the polymer namelygelatin. Although, limited research has been carried outto understand the interaction between gelatin and noble metal such as silver and gold nanoparticles, their use especially in biomedical applicationsis not well studied [9]. |
| In the present investigation, gelatin derived from collagen extracted from chrome containing leather waste (CCLW) has been used as a template to synthesize Ag, Au and bimetallic Ag-Au nanoparticles. Gelatin acts as a reducing agent and also performs the role of a stabilizer in the nanoparticle synthesis. The air dried nanobiocomposites of gelatin directed bimetallic nanoparticles showed increased thermal stability, a parameter useful for biomedical applications. Also, these scaffolds possessed desired properties such as increased tensile strength along with water absorption capacity suitable for tissue engineering applications. Additionally, the in vitrostudieswere carried out to evaluate the biocompatibility of these gelatin based scaffolds. |
MATERIALS AND METHODS |
a) Isolation of collagen from chrome containing leather waste (CCLW) |
| Collagen was extracted from the chrome containing leather waste following the procedure reported by Mandal et al. [18]. The CCLW was dechromed using concentrated sulphuric acid.The dechromed sample was then treated with 0.1M Tris HCl (pH 8), 0.2M β- Mercaptoethanol, 0.05M EDTA for 3 days. The collagen fibrils were suspended in 0.05M acetic acid with a sample/solution ratio 1:30 (w/v) containing pepsin 1:10,000 (w/w) at 4°C for 24 h. The pepsin solubilized collagen was centrifuged at 10000 rpm (7155 g) for 1 h. The supernatant was further dissolved in 0.05 M acetic acid; subsequently dialyzed and the collagen solution was stored for further use and analyses. |
b) Synthesis of silver nanoparticles (AgNPs) |
| Silver nanoparticles (AgNPs) were prepared using collagen extracted from CCLW. The aqueous collagen solution with different solid concentrations (100 mL) was heated to 80±3°C. Collagen when heated to 80±3°C denatures to gelatin which acts as reducing/stabilizing reagent. 2 mL of the AgNO3 solution (0.4 wt%) was added rapidly at a stirring rate of 3000 rpm . A color change from pale yellow to white was observeddue to the complex formation between gelatin and Ag+ ion [9]. The reaction was carried out under dark conditions and the contents were subjected to vigorous stirring for 10 h at 80±3°C to ensure the complete formation of gelatin capped silver nanoparticles. |
c) Synthesis of Gold Nanoparticles (AuNPs) |
| The aqueous gelatin solution with different solid concentrations (100 mL) was heated to 80±3°C.2 mL of HAuCl4 solution (0.4 wt%) was added rapidly under vigorous stirring. The contents were further stirred for 4 h at 80±3°C, which resulted in purplish red gelatin-AuNPs solution. |
d) Synthesis of bimetallic nanoparticles (Ag–AuNPs) |
| Silver–gold nanoparticles (Ag–AuNPs) were prepared using gelatin as reducing/stabilizing reagent. The aqueous gelatin solution (20 mL) was heated to 80±3°C. 10 mL of 0.01 M AgNO3 was added to gelatin solution and stirred at 3000 rpm. Subsequently,10 mL of 0.01M HAuCl4 solution was added to the contents and stirring continued for 5 h. The generation of purple color indicates the formation of gelatin-Ag-AuNPs. |
RESULTS AND DISCUSSIONS |
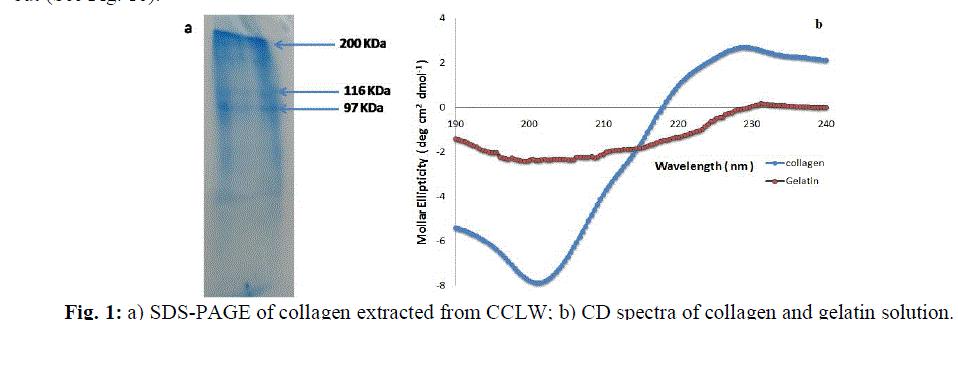
| The collagenextracted from the chrome leather waste was confirmed by SDS-PAGE.The SDS-PAGE results shown in Fig.1ademonstratea distinct β band in addition to characteristic α bands (α1 and α2), which were the unfolding polypeptide chains of collagen triple helix [19]. This confirms the triple helical nature of the collagen. Also, the circular dichroism (CD) spectra of both collagen and aqueous gelatin solution when heated at 80±3°C were carried out (See Fig. 1b). |
 |
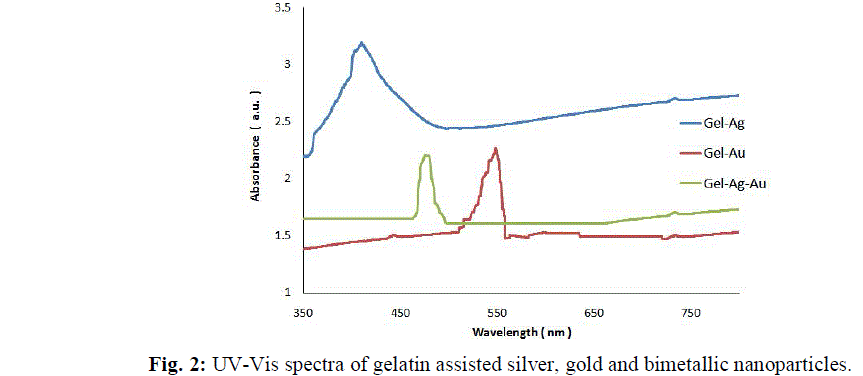 |
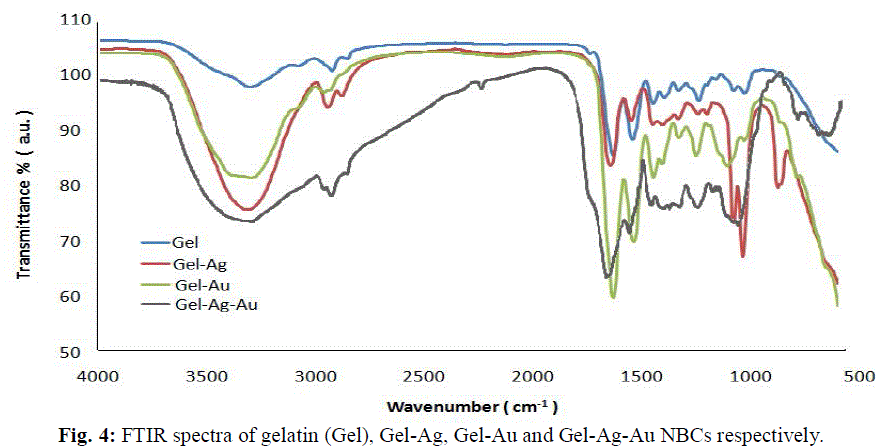
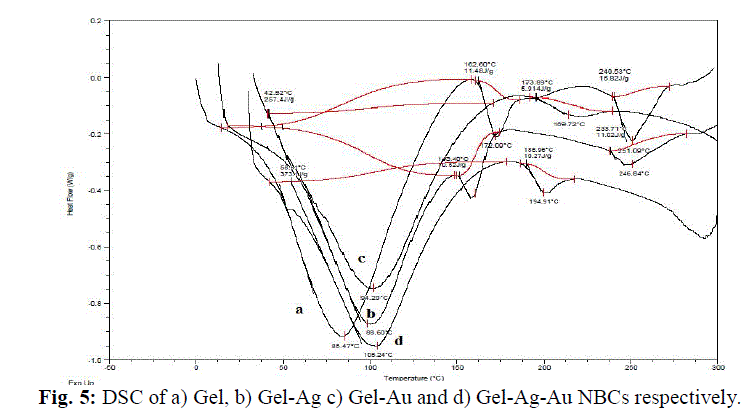
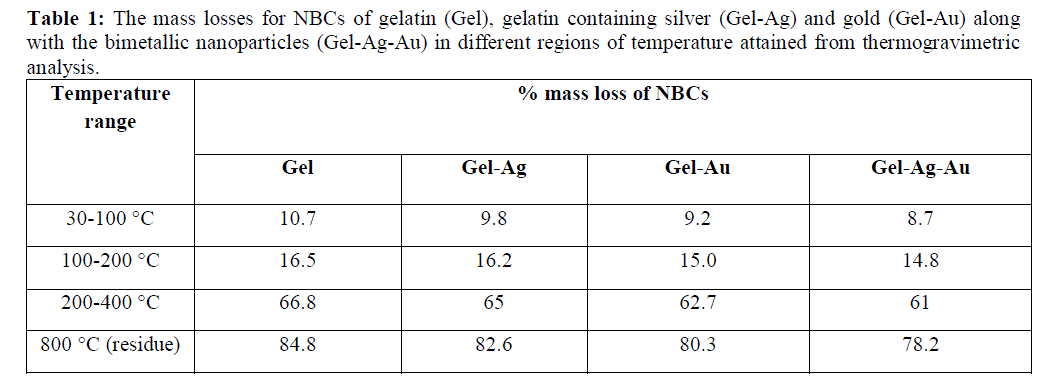
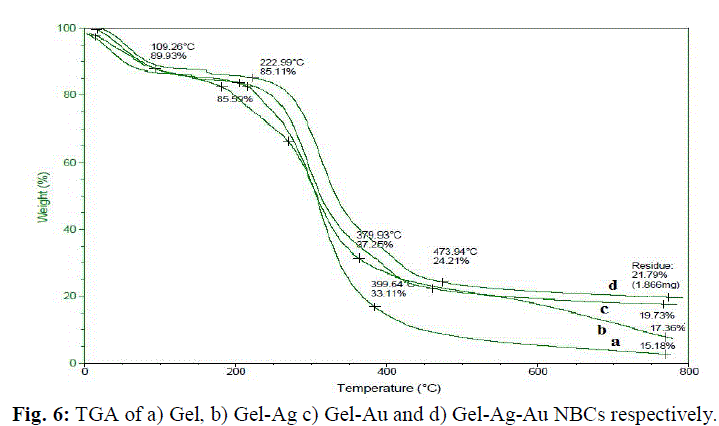
| The gelatin based NBC containing bimetallic Ag-Au NPs exhibited the maximum peak shift. Also, a peak at 1550 cm-1due to the NH3 + group in the gelatin was noted. The adsorption of Ag, Au and the bimetallic Ag-Au NPs on the surface of gelatin in the NBCs resulted in the peak shift to 1560, 1558 and 1555 cm-1 respectively. This indicates the interaction of synthesized NPs with that of gelatin. Also, the peaks noticed at 1087 and 1041 cm-1 due to vibrations of the C-C bonds of the gelatin is red shifted with the incorporation of the nanoparticles in the gelatin matrix. The maximum peak shift is observed in gelatin NBC containing bimetallic Ag-Au NPs. The DSC investigations showed three endothermic peaks at 85, 152 and 251 °C were observed for gelatin NBC (see Fig. 5). |
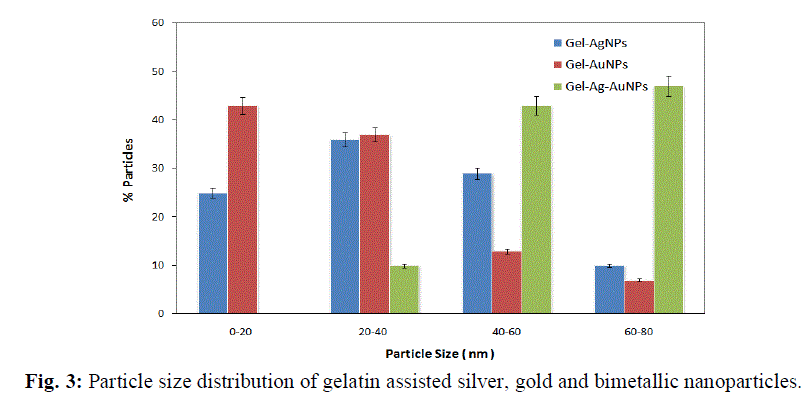 |
 |
| The surface morphology of the prepared scaffolds analyzed using SEM is depicted in Fig.8. The SEM images reveal the homogenous and uniform distribution of Ag and Au NPs in the gelatin scaffolds. Similarly, uniform distribution with very little aggregation was observed when bimetallic (Ag-Au) NPs were incorporated onto gelatin matrix. This has resulted in smooth surface of the scaffolds which is essential and a pre requisite of a material to be used for wound dressing purposes. It is well known that higher concentrations of gelatin results in the aggregation of the nanoparticles [4]. Therefore, in the present investigation, the bimetallic Ag-AuNPs and gelatin were mixed in the ratio 1:1 (v/v) to restrict the aggregation of the synthesized nanoparticles. The EDX spectra exhibit a strong signal of silver along with gold atoms which confirms the presence of both silver and gold nanoparticles in the gelatin scaffolds. Additional signals of C and O atoms observed in the gelatin scaffold are attributed to the carbon moieties and carboxyl groups present in the gelatin. |
 |
| where Wd and Wh are the initial dry weight and the hydrated weight of the NBC observed at different time periods, respectively. |
| Moreover, the porosities of the fabricated NBCs were obtained from the procedure followed by Shimizu et al. [25] and calculated using the following equation: |
 |
| The water absorption capacity (WAC)or the swelling ratio of the NBCshas significant importance as those NBCs with better WAC when applied on an open wound surface can easily absorb the exudates and allows the wound surface to remain dry and safe from air borne infections [26]. In the present investigation, all the NBCs exhibited high swelling ratio because of increase in water absorption and this increasedwith time with the maximum value attained at 24 h (See Fig.9). Also, it was noted that the presence of nanoparticles; both silver and gold in the gelatin NBC resulted in the lower swelling ratio (690±15%) due to decrease in water uptake. This is probably due to the fact that gelatin assisted template synthesis of nanoparticles resulted in their incorporation in the pores of the gelatin and thereby exhibited lower capacity to absorb water. The porosity of gelatin NBC was found to be 89% whereas NBCs when incorporated with nanoparticles resulted in decreased porosity (81 and 78% for Ag and AuNPs) and was least in the case of bimetallic Ag-Au NPs (71%). Thus, the gelatin NBC containing bimetallic Ag-Au nanoparticles displayed the least WAC as a porous NBC can take up and store more water compared to the non-porous ones. The result indicates that the developed NBCs are biodegradable and have the capacity to absorb water required for implantation applicationsespeciallyas materials for wound dressings. |
 |
| This finding may be attributed to the high affinity of NIH3T3 cells to collagen and their migration around the NBCs. Moreover, it has been demonstrated that the presence of nanoparticles especially silver modulates the alignment of collagen and augment the fibroblast cell proliferation and its differentiation [27]. Here, in our case, the in vitro results suggest that the bimetallic(Ag-Au) nanoparticles impregnated in the gelatinNBC perform this function more effectively and can be used as scaffolds which are vital for clinical wound healing applications. |
CONCLUSIONS |
| Gelatin obtained from collagen extracted from CCLW serves as a template for the synthesis of Ag, Au as well as bimetallic Ag-Au nanoparticles and this green approach can be utilized to develop value added NBCs. The gelatin based NBCcontaining bimetallic Ag-Au NPs is biodegradable and possess enhancedstability; desired mechanical properties in addition to excellent biocompatibility demonstrated by the in vitro studies that can be used as scaffolds for various potential biomedical applications. Further in vivo studies to ascertain the validity of these gelatin based NBCs as wound dressing materials are required and work is being carried out in this direction. |
ACKNOWLEDGEMENTS |
| We would like to thank Prof. Dr. A. B. Mandal for allowing us to carry out the experiments in CSIR-CLRI institute. |
References |
|