E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
Mingliang Zhang, Feng Qi, Xianzhang Jiang, You Weichen, Weibin Wu, Xiaolei Guo and Jianzhong Huang*
Engineering Research Center of Industrial Microbiology, College of Life Sciences, Fujian Normal University, Fuzhou, P.R. China
Received date: 13/03/2017; Accepted date: 21/03/2017; Published date: 27/03/2017
Visit for more related articles at Research & Reviews: Journal of Microbiology and Biotechnology
A set of kinetic models were established for the fed-batch production of DHA by Schizochytrium sp. FJU-512 in 151 and 1001 fermenters using a strategy that applies nitrogen stress in combination with shifts of DO concentration and temperature. A compensatory parameter n was integrated into the model in order to find the optimal mathematical equations. A modified Logistic model was proposed to fit the cell growth data, and the following kinetic parameters were obtained: μm=0.0525 h-1, Xm=100 g l-1 and n=4.1717 for the 151 bioreactor, as well as μm=0.0382 h-1, Xm=107.4371 g l-1 and n=10 for the 1001 bioreactor. The Luedeking-Piret equations were utilized to model DHA production, yielding values of α=0.0648 g.g-1 and β=0.0014 g.g-1.h-1 for the 151 bioreactor, while the values of α and β obtained for the 1001 fermentation were 0.0209 g.g-1 and 0.0030 g.g-1.h-1. The established models had a good fitting precision and were able to exactly depict the dynamic features of the DHA production process which utilizes Schizochytrium sp. FJU-512.
Schizochytrium sp. FJU-512; Docosahexaenoic acid; Fed-batch; Logistic model; Luedeking model
Docosahexaenoic acid (DHA, C22:6) is an omega-3 polyunsaturated fatty acid that is essential for human health, as it is a major structural component of the central nervous system, retina and heart tissue [1]. Furthermore, it is a well-known polyunsaturated fatty acid of great economic value, due to its wide use in food-related industries and its potential applications in medicine [2]. In fact, DHA has been investigated in the prevention and treatment a wide range of diseases, including heart disease, high blood pressure [3], inflammation [4], cancer [5], and Alzheimer's disease [6].
Marine fungoid protists such as Schizochytrium sp. are known to be excellent DHA producers [7]. However, a great number of variables affect DHA production by Schizochytrium sp., such as medium composition [8], nitrogen limitation [9], temperature [10] and oxygen supply [11], which makes efficient industrial production challenging. Generally, the cells’ lipid content can be enhanced under specific cultural conditions, such as low temperature [12], low oxygen levels [13] and nitrogen depletion [14]. However, such unfavorable conditions also depress biomass yields, affecting overall process productivity. To circumvent this limitation, we developed a nitrogen limitation, DO-shift and temperature-shift strategy which enables efficient DHA production using Schizochytrium sp. FJU-512. The strategy includes an initial phase with sufficient nitrogen, in combination with high DO and temperature to ensure adequate biomass accumulation. Establishing a mathematical model of this process can provide very useful information for the scale-up and the implementation of industrial cultivation processes which utilize Schizochytrium sp. for high-quality DHA production. Although many studies have dealt with the kinetics of cell growth and mathematical modeling of fermentation processes [15-18], the kinetics of DHA production by Schizochytrium sp. in batch and fed-batch fermentation processes are still quite scarce. Song et al. developed a simple batch fermentation model which uses the logistic equation for growth and the Luedeking-Pirt-like equation for DHA production and substrate consumption by Schizochytrium limacinum OUC88 [19]. Surendhiran et al. found that nitrogen was an essential factor for algal growth, and they provided kinetic models which simulate the growth and lipid production of two marine microalgae under both nitrogen-replete and nitrogen-depleted conditions, using logistic and Luedeking-Piret equations [20]. It is necessary to investigate the kinetics of pilot-scale fed-batch cultures, since control models of high-cell-density fermentations for enhanced DHA production are difficult to obtain due to the addition of a liquid matrix to the fermentation medium.
In this study, the fermentation kinetics of DHA production by Schizochytrium sp. FJU-512 were studied in fed-batch processes at different scales, and the microbial growth and DHA production dynamics were recorded. We propose a modified model, which employs the logistic equation for growth and the Luedeking-Piret-like equations for DHA production.
Microorganism
Schizochytrium sp. FJU-512 was used for all experiments. It was originally isolated using the pine pollen baiting technique.
Medium
A basal medium comprising 30 g/l glucose, 10 g/l peptone, 5 g/l yeast extract and 15 g/l sea salts was used for routine cultivation. The fermentation medium contained 30 g/l of glucose, 2.5 g/l (NH4)2SO4, 15 g/l yeast extract , 15 g/l peptone, 25 g/l sea salts, 2 g/l KH2PO4, 3 g/l Na2SO4, 0.005 g/l vitamin B1, and 0.005 g/l vitamin B12.
Inoculum Preparation
Static cultures of Schizochytrium. sp FJU-512 were grown for 2 days at 28°C and used to inoculate 1 l flasks containing 400 ml of basal medium. The resulting cultures were incubated at 28°C under constant orbital shaking at 230 rpm for 2 days.
Fed-Batch Cultures
The fed-batch cultures were carried out in a 151 and a 1001 bioreactor (Zhenjiang East Biotech Equipment and Technology Co., Ltd., China), respectively. Culture conditions such as temperature, pH, DO and agitation rate were controlled automatically. We started the cultivation with initial volumes of 8 l in the 151 fermenter and 551 in the 1001 fermenter. Subsequently, 10 g/l organic nitrogen source, 20 g/l sea salts and 80% glucose were added continuously into the fermenters when the residual glucose was below 2% after 24 h. Fed-batch cultures were performed at 28°C in bioreactors before mid-exponential phase, after which the temperature was lowered to below 28°C to enhance the accumulation of lipids. The initial aeration rate was 1 vvm and was subsequently adjusted manually. pH was maintained at 5.7 ± 0.1 by automated addition of 28% industrial ammonia.
Biomass Determination
A 10 ml cell suspension sample was taken from the culture and centrifuged at 8000 rpm for 5 min. The cell pellets were rinsed with deionized water and centrifuged under the same conditions. This procedure was repeated 2-3 times. The washed cells were dried at 85°C for 24 h to obtain the dry biomass used to determine the cell dry weight.
Lipid Extraction
Dried cell samples prepared same as above were used directly for lipid extraction. The cell pellets were dissolved in 5 ml 6 M HCl and incubated for 1h at 80°C, after which the lipids were extracted using 2 ml n-hexane and 0.75 ml absolute ethyl alcohol. The mixture was blended by vigorous vortexing and centrifuged at 800 Xg for 2 min to improve phase separation. The extraction process was repeated 3 times to collect the greatest possible amount of lipids. The total lipids were determined gravimetrically after evaporation of the solvents under oxygen-free nitrogen on a heating block maintained at 50°C.
Analysis of Fatty Acid Composition
An aliquot comprising 100 mg of lipids was transmethylated at 62°C for 1h using 2 ml 10% (v/v) methanolic HCl. Subsequently, the fatty acid methyl esters (FAMEs) were dissolved in 2ml n-hexane. GC-MS (Agilent 6890 N/5975 C) analysis of FAMEs was conducted using an HP-INNOWAX capillary column (30 m × 0.25 mm × 0.25 μm). High purity helium (99.999%) was used as the carrier gas at a flow rate of 1.0 mL/min. The injector temperature was maintained at 250°C, and a volume of 1 μl was injected using the split mode at a ratio of 5:1. Column temperature was raised from 150 to 220°C at 10°C/min and further to 230°C at 2°C/min, where it was kept for 5 min. All fatty acids were identified by comparing to the NIST mass spectral library (Agilent Technologies, USA). The mass spectroscopy instrument was operated at an ionization voltage of 70 ev, with a scan range of 35- 450 amu. Concentrations of the fatty acids were calculated based on the total peak areas compared to an internal standard, as published previously [21].
Defining the Kinetic Parameters
In order to prevent the inhibitory effects of high glucose concentrations in the cultivation process, and obtain maximum DHA production, we chose fed-batch cultures to obtain kinetics, estimate models, and calculate parameters. Kinetic parameters were calculated according to the following equations:
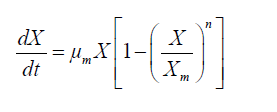 (1)
(1)
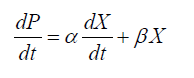 (2)
(2)
The logistic constants for cell growth were evaluated using the cftool kit in MATLAB version 7.1. Equations 1 and 2 were solved by numerical integration, and ode45 solver version was employed for fitting of the results.
Effects of Fermentation Conditions on Schizochytrium Sp. Growth and DHA Production
In general, glucose was utilized by Schizochytrium to produce DHA under oxygen-limiting and nitrogen-limiting conditions. We performed experiments in several stages to increase the biomass concentration and promote DHA production. The fermentation process of Schizochytrium can be divided into two stages [22]: stage 1, in which cell numbers and biomass increase with little accumulation of lipids, and stage 2, in which in which cell size enlarges due to lipid accumulation and DHA synthesis. At first, the cells grew rapidly with fast glucose consumption under adequate nitrogen and oxygen supply, whereby the dissolved-oxygen tension (DOT) was maintained above 20% of air saturation between 0h and 48 h by manually controlling the stirrer speed (range: 200-465 rpm) and aeration rate (air volume/culture volume/min, range: 1-2 vvm). The pH was kept at 5.7 ± 0.1 by automated addition of 28% industrial ammonia at the first 60 h, and the cultivation temperature was set to 28°C during the first 96 h. In the second stage, the cells expanded as intracellular lipids accumulated, but the number of cells did not increase much between this stage and the end of fermentation. It is known that a high carbon-nitrogen ratio, low DO and low temperature are essential for lipid accumulation and DHA production in oleaginous microorganisms [12-14]. Therefore, it was not necessary to adjust the agitation and aeration rates, and DO was limited within 5%. Subsequently, 80% glucose and 10 g.l-1 organic nitrogen were added continuously to ensure an excess supply of carbon source and nitrogen, and this was done without pH regulation. After 96h, the temperature was decreased from 28°C to 25°C to enhance DHA accumulation. The results of this control strategy revealed that DHA content increased from 44.1% to 51.5% with reduced DO after 36 h (Table 1). When available carbon was constantly increased and nitrogen was limited, which occurred from 60 h to the end of fermentation, the lipid content increased from 25.8% to 29.4%. DHA productivity was 133.7 mg.l-1.h-1 in the fed-batch fermentation after 120 h, and DHA content increased from 42.2% to 44.7% with changing cultivation temperature. However the DHA concentration experienced a slight decrease when we reduced the temperature (Table 1). The highest cumulative production at 120 h was achieved using the feeding strategy, with DCW, total yield and DHA production reaching 103.9 g.l-1, 37.2 g.l-1 and 16.0 g.l-1, respectively (Table 1).
| Time(h) | Biomass | Total lipids (g.l-1) | DHA (g.l-1) | TFA (%) | DHA (%) |
|---|---|---|---|---|---|
| 36.00 | 13.67 ± 0.82 | 2.47 ± 0.12 | 1.09 ± 0.05 | 18.07 ± 0.41 | 44.13 ± 0.63 |
| 48.00 | 39.16 ± 2.35 | 8.72 ± 0.44 | 4.49 ± 0.22 | 22.27 ± 0.57 | 51.49 ± 0.91 |
| 60.00 | 64.50 ± 2.87 | 16.65 ± 0.96 | 7.92 ± 0.43 | 25.81 ± 1.29 | 47.57 ± 1.50 |
| 72.00 | 76.40 ± 3.58 | 22.44 ± 1.12 | 10.57 ± 0.53 | 29.37 ± 0.11 | 47.10 ± 1.72 |
| 84.00 | 88.22 ± 3.29 | 25.92 ± 1.30 | 10.70 ± 0.53 | 29.38 ± 0.93 | 41.28 ± 0.82 |
| 90.00 | 88.40 ± 3.30 | 27.04 ± 1.35 | 11.68 ± 0.58 | 30.59 ± 1.67 | 43.20 ± 1.65 |
| 96.00 | 88.72 ± 2.32 | 31.16 ± 0.96 | 13.16 ± 0.66 | 35.12 ± 0.57 | 42.23 ± 0.91 |
| 108.00 | 96.36 ± 3.78 | 32.88 ± 1.14 | 14.71 ± 0.74 | 34.12 ± 1.29 | 44.74 ± 1.50 |
| 120.00 | 103.86 ± 3.23 | 37.18 ± 0.86 | 16.04 ± 0.80 | 35.80 ± 0.84 | 43.14 ± 0.60 |
Table 1: Biomass, TFA and DHA contents obtained in the fed-batch culture of Schizochytrium sp. FJU-512 in the 15l bioreactor.
The results obtained in the present study thus indicate that DHA production by Schizochytrium sp. FJU-512 is greatly influenced by the availability of nitrogen. Although nitrogen limitation is a prerequisite for lipid accumulation in oleaginous microorganisms, a high C/N ratio leads to a decrease in biomass [21]. The regulation of lipid biosynthesis in oleaginous microorganisms requires a sufficient supply of NADPH as reductant, while under conditions of nitrogen exhaustion the activity of AMP deaminase is up-regulated, which results in the formation of acetyl-CoA [23]. Surendhiran et al. reported that under nitrogen-limited conditions, the lipid content of microalgae increased by shifting the metabolic fluxes from the protein and carbohydrate pathways [20].
In addition, temperature was another main factor that affected the accumulation of biomass and DHA. Zeng et al. obtained the highest DHA content of 51.98% (per total fatty acids) and DHA production of 6.05% (per dry cell weight) in Schizochytrium HX-308, using the strategy of shifting the cultivation temperature from 30°C for 32 h to 20°C for 12 h in shake flasks [12]. Pasquet et al. found that microalgal cell membrane fluidity increases as an adaptation to low-temperatures [24]. Furthermore, it has been reported that only the intracellular lipids and organelle membranes were influenced by the cultivation temperature [25]. In our work, we used a temperature shift from 28 to 25°C. In the first stage, the culture temperature was set at 28°C in order to generate sufficient biomass. In the lipid accumulation stage, the temperature was lowered to 25°C, and the DHA content increased significantly, even though the cells grew slowly. Thus, this simple temperature shift strategy is an effective way to obtain enhanced biomass and DHA accumulation from Schizochytrium. sp FJU-512.
Furthermore, a high oxygen supply does not necessarily result in more DHA production in Schizochytrium sp. Qu et al. showed that an increased KLa of 150 h−1 provided conditions favorable for cell growth, while a KLa that was controlled at 88.5 h−1 was able to effectively improve the biosynthesis of DHA [26]. It is known that Schizochytrium uses a PKS-like system to synthesize PUFAs, in which acetyl-CoA and malonyl-CoA are utilized as the initial building blocks [27]. An anaerobic culture system was needed for DHA synthesis from saturated lipids in oleaginous microorganisms. Thus, a strategy of shifting the growth conditions from high to low oxygen concentration was developed to increase the DHA production in this study. Using fed-batch culture, combined with a temperature and oxygen-concentration shift strategy, Schizochytrium grown in the 151-reactor reached a biomass of 103.9 g.l-1, a total lipid yield of 37.2 g.l-1, and a DHA yield of 16.0 g.l-1. By contrast, the highest biomass, lipid and DHA values obtained during fermentation in the 1001 reactor were 110.8 g.l-1, 50.8 g.l-1 and 22.3 g.l-1, respectively. Thus, both the temperature and oxygen shift strategies have been demonstrated as effective methods to enhance DHA production by Schizochytrium sp. FJU-512 in 151 and 1001 bioreactors.
Microbial Growth
According to a study by Song et al, DHA synthesis is associated with the microbial growth rate [28]. Qu et al. indicated that fed-batch cultivation was a more efficient manipulation compared to batch fermentation [29]. A simple kinetic and logistic equation was given in this study to describe the cell growth of Schizochytrium sp. FJU-512 in the fed-batch process, calculated using MATLAB 7.1. Time courses of cell growth (Figures 1 and 2) were plotted by fitting sigmoidal growth data to the integrated equation. In order to study the kinetics and obtain a good fitting effect of the model, some assumptions were required: (1) the concentration of glucose was high enough for cell growth and DHA biosynthesis; (2) the dissolved oxygen was sufficient and was not a limiting factor for the growth rate during the entire fermentation process; (3) the feeding volume was equal to the volume of evaporation and sample withdrawal, so that the biosynthetic process could be regarded as having a constant volume; (4) the similarity of structure and dimensions of the experimental fermenters (heat and mass transfer coefficients) are consistent. However, there were large differences between the 151 and 1001 stirred tank bioreactors. Here, the parameter n was used to add model compensation in order to find the optimal mathematical equations.
Interestingly, a lag phase was observed at 24-36 h in both the 151 and the 1001 fermenter, suggesting that biomass accumulation was inhibited by the substrate. However, carbon and nitrogen sources were consumed quickly during the exponential growth phase. Thus, the concentrated medium that was added after the lag phase comprised 80% glucose and 10 g.l-1 nitrogen source, in order to avoid unnecessary dilution. Subsequently, the cell growth kinetics of Schizochytrium sp. FJU-512 during fed-batch cultivation and the multi-stage control strategy in the 151 and 1001 fermenters were analyzed and simulated in accordance with the results. The integrated logistic model was established to fit the cell growth kinetics. The value of μm was 0.0525 h-1, Xm was 100 g.l-1 and n was 4.1717. These values were obtained from the parameter estimation with a relative error of 0.1160 in the 151 bioreactor. Correspondingly, μm, Xm and n were 0.0382 h-1, 107.4371 g.l-1 and 10, respectively, with a relative error of 0.0810 in the 1001 fermenter. The value of μm in the 151 fermentation process was less than the same parameter of the 1001 cultivation mainly because there was a much longer time lag (36 h) in the 1001 fed-batch culture than in the former (24 h). The Xm of the 1001 fermenter was greater than that of the 151, which was attributed mainly to the better efficiency of dissolved oxygen transfer in the larger fermentation tank. It was therefore assumed that the growth follows logistic kinetics as follows:
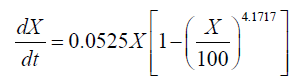 (3)
(3)
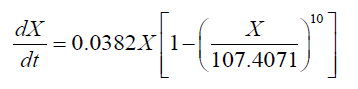 (4)
(4)
DHA Production
The relationships between the rate of product formation and cell growth can be divided into 3 types: the growth-associated model, semi-growth-associated model and no-growth-associated model [29]. In this case, increased DHA production was associated with enhanced lipid and biomass accumulation. The results thus indicated that DHA production could be considered a semi-growth-associated process. Figures 3 and 4 show the kinetics of the DHA yield in the 151 and 1001 fed-batch reactors, based on the Luedeking-Piret model. The parameters α and β for the cultivation in the 151 stirred tank obtained using the model were 0.0648 g.g-1 and 0.0014 g.g-1.h-1, respectively. The final formula describing the DHA production kinetics in the 151 bioreactor was:
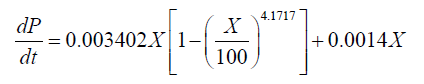 (5)
(5)
By contrast, the values of α and β obtained for the 1001 fermentation were 0.0209 g.g-1 and 0.0030 g.g-1.h-1, respectively. The corresponding DHA production model was described as:
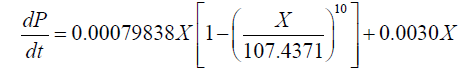 (6)
(6)
Therefore, the results demonstrate that the value of α was much higher than β in each case, suggesting that cell growth had a major effect on DHA production in Schizochytrium sp. FJU-512.
Our results demonstrate that the culture-condition shift strategy developed in this study constitutes an effective method to enhance DHA production by Schizochytrium sp., even in scaled-up processes. While the μm value in the 151 fed-batch fermenter was higher than in the 1001 reactor, the Xm value showed the opposite trend. Both values had the same number of levels, which was mainly due to the modification of the parameter n. The relevant errors were 11.60% and 8.10%, respectively, indicating that the modified logistic and Luedeking-Piret model equations were able to provide satisfactory fitting for the kinetics of DHA production by Schizochytrium sp. FJU-512 in fed-batch systems at different scales. The fitting results also revealed that the parameter α was greater than β, which demonstrated that DHA production was highly dependent on cells growth, in agreement with the literature [20]. Furthermore, the established models had a good fitting precision and were able to exactly depict the dynamic features of the DHA production process which utilizes Schizochytrium sp. FJU-512. Therefore, the mathematical modeling of Schizochytrium sp. fermentations could be an important development and analysis tool for the optimization of biomass accumulation and DHA production.
This work was financially supported by the National Natural Science Foundation of China (No. 21406130). We sincerely thank Professors Chun Men and Hang Wang (Fuzhou University, Fuzhou, Fujian 350108, PR China) for providing the MATLAB program.