e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
Murari Pavan1*, Prasanth VV1, Abhishek Sharma1, Ajay Chauhan1, Rinku Mathappan2, and Sam T Mathew3
Department of Pharmaceutics, Gautham College of Pharmacy, Bangalore 560 032, Karnataka, India.
Department of Pharmacognosy, Gautham College of Pharmacy, Bangalore, Karnataka, 560 032, India.
Regulatory Affairs and Medical Writing, Biocon Pvt Ltd, Bangalore 560 100, India.
Received date: 11/01/2014 Accepted date: 05/03/2014 Revised date: 27/02/2014
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
The purpose of this research work was to develop gastroretentive drug delivery system of Dicloxacillin sodium. Floating tablets were prepared by wet granulation method using gas generating agents such as sodium bicarbonate and citric acid anhydrous, like Hydroxypropyl methylcellulose (HPMC K100M), Xanthan gum, Sodiumcarboxy methylcellulose (NaCMC) and Microcrystalline cellulose (MCC). Dicloxacillin sodium floating tablets were prepared by wet granulation method were found to be good without chipping, capping and sticking. The drug content was uniform in all the tablet formulations indicating uniform distribution of drug within the matrices. All the prepared batches showed satisfactory floating lag time and total floating time found to be more than 12 h. Formulation F14 showed desired drug release selected as a best formulation and subjected to stability studies for 3 months showed that formulation is intact without interaction. Finally optimized formulation F14 complying with all properties of floating tablets and found to be satisfactory.
Floating tablets, In vitro Buoyancy study, Drug content, Water uptake study
The oral route is the most preferred route of administration because of its patient compliance. Now a day’s controlled systems are designed offering a number of advantages including improvement in reduced dosing frequency, therapeutic efficacy, safety and patient compliance. Gastric retention time is one of the important factors, which adversely affect the performance of these drugs when administered simply by an oral controlled drug delivery system [1].
Dicloxacillin sodium is a narrow-spectrum beta-lactam antibiotic of the penicillin class and having more acid-stable than many other penicillins and can be given orally. It is used for the treatment of pneumonia, bone, ear, skin and urinary tract infections caused by susceptible Gram-positive bacteria. It is active against β-lactamase producing organisms such as Staphylococcus aureus, which would otherwise be resistant to most penicillins. β-lactam antibiotics were mainly active only against Gram-positive bacteria, these will work by inhibiting cell wall biosynthesis in the bacterial organism [1].
The absorption of Dicloxacillin sodium after oral administration is rapid but incomplete. Peak blood levels are achieved in 1 - 1.5 h. It indicates variable absorption from the gastrointestinal tract. Dicloxacillin sodium has a bioavailability of 60-80 % and half-life of less than 1 h. Thus, this necessitates frequent administration of Dicloxacillin sodium. So, to overcome these problems (absorption, half-life of the drug and to reduce the dosing frequency) gastro retentive floating drug delivery system of Dicloxacillin sodium were prepared provide satisfactory drug release, maintain a constant blood levels and prolong the duration of action.
The floating tablets were prepared by wet granulation method [2], using different hydrophilic and hydrophobic polymers HPMC K100M, Xanthan gum, Na CMC and MCC different ratios of polymers is prepared as mention in Table 1. The ingredients were weighed accurately and mixed thoroughly. The granulations were done with starch paste (22 mesh). The granules were dried in conventional hot air oven at 45 °C. Drying of granules was stopped when the sample taken from the oven reached a loss on drying (LOD) value of 1 – 3%, as measured by a moisture balance at 105°C. The dried granules were sized through 40 / 60 mesh, lubricated with magnesium sterate (1% w / w) and purified talc (1% w / w) and then compressed. The tablets were prepared by using a rotary tablet compression machine (12mm diameter, Riddhi 10 stn mini tablet press RDB-10, Rimek, Ahmedabad, India).
Bulk density is a ratio of mass of powder to bulk volume. The bulk density depends on particle size distribution, shape and cohesiveness of particles. Accurately weighed quantity of powder was carefully poured in to graduated 100 mL measuring cylinder through large funnel and volume was measured, which is called initial bulk volume. It is expressed in gm / mL and is given by [3]
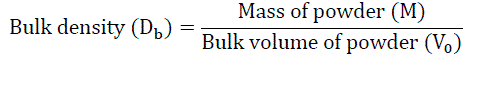
Accurately weighed quantity of powder was carefully poured in to graduated 100 mL measuring cylinder through large funnel. The cylinder was then tapped 100 times from a constant height and the tapped volume was read. It is expressed in gm/mL and is given by [3]
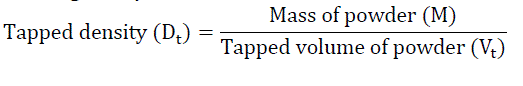
It is defined as the maximum angle possible between the surface of the pile of the powder and the horizontal plane. Fixed funnel method was used, a funnel was fixed with its tip at a given height ‘h’ above a flat horizontal surface to which a graph paper was placed. Powder was carefully poured through a funnel till the apex of the conical pile just touches the tip of the funnel. The angle of repose was then calculated using following equation[3].
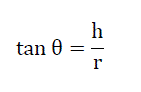
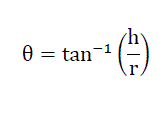
Where, θ= angle of repose
h= height of the pile and
r= radius of the powder cone
Carr’s index is an indication of the compressibility of a powder. It is expressed in percentage and is given by [4]

A small index like percentage compressibility index has been defined by Hausner. Values less than <1.25 indicates good flow, where as greater than 1.25 indicates poor flow. Added glidant normally improves flow of the material under study. Hausener’s ratio can be calculated by [4],
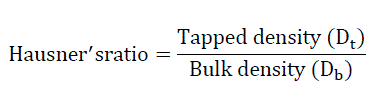
Thickness and diameter were tested in 5 different randomly selected individual tablets from each batch. The thickness and diameter of tablets were measured by digital vernier calipers [5].
Hardness (diametric crushing strength) is a force required to break a tablet cross the diameter. The hardness of a tablet is an indication of its strength. The tablet should be stable to mechanical stress during handling and transportation. The degree of hardness varies with the different manufactures and with the different types of tablets. The hardness was tested by using Monsanto hardness tester. The averages of five determinations were taken5.
Weight variations were tested in 10 different randomly selected individual tablets from each batch. Weight variations were measured by digital electronic balance (Citizen D 1262, India). The averages of ten determinations were taken; weight variation can be calculated by [5].
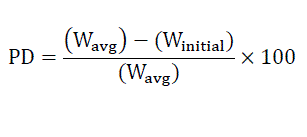
Where PD= Percentage deviation,
Wavg= Average weight of tablet,
Winitial= Individual weight of tablet.
Friability is the loss of weight of tablet in the container/package, due to removal of fine particles from the surface. This in process quality control test is performed to ensure the ability of tablets to withstand the shocks during processing, handling, transportation, and shipment. Permitted friability limit is 1.0 %. Roche friabilator (Ketan, Mumbai) was used to measure the friability of the tablets. Ten tablets were weighed collectively and placed in the chamber of the friabilator. In the friabilator, the tablets were exposed to rolling, resulting free fall of tablets (6 inches) within the chamber of the friabilator. It was rotated at a rate of 25 rpm. After 100 rotations (4 minutes), the tablets were taken out from the friabilator and intact tablets were again weighed collectively [5].
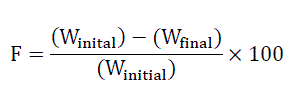
The in vitro buoyancy was characterized by floating lag time and floating duration. The test was performed using USP type II paddle type apparatus using 900 ml of 0.1 N HCl at paddle rotation of 50 rpm at 37±0.50. The floating lag time (time period between placing the tablet in the dissolution medium and tablet floating) and floating duration of the tablets were determined by visual observation [6].
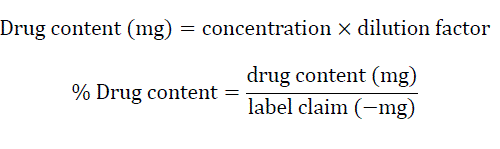
Ten tablets were crushed and powdered. Weighed accurately the quantity equivalent to 100 mg of drug and taken in 100 mL volumetric flask and dissolved with small quantity of 0.1N HCl (pH 1.2) and volume made up to the mark with same medium and stirred for 12 hrs. After stirring, 1 mL solution was withdrawn and filtered through 0.45 μm Whatman filter paper and volume made up to 10 mL of water. The absorbance was measured and at 263 nm using UV Spectrophotometer (Shimadzu 1800, Japan) [7].
The swelling behaviour of a dosage units were measured by studying its weight gain. The swelling index of tablets were determined by placing the tablets in the basket of dissolution apparatus using dissolution medium 0.1N HCl at 37 ± 0.5 °C. After 1, 2, 3, 4 and 5 h, each dissolution basket containing tablets were withdrawn and blotted with tissue paper to remove the excess water and weighed by digital electronic balance (Citizen D 1262, India). Swelling index was calculated by using following formula [7].

In vitro drug release studies were carried out using USP dissolution apparatus II (Paddle model, TDL 084, Electrolab, India). The dissolution studies were performed using 900 mL of 0.1N HCl (1.2 pH) at 37 ± 0.5 °C at 50 rpm. The sample (1 mL) was withdrawn at predetermined time intervals (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 h) and replaced with same volume of fresh dissolution medium. The withdrawn sample (1 mL) was diluted with 10 mL of distil water, filtered through 0.45 μm Whatman filter paper and assayed by using UV Spectrophotometer (Shimadzu 1800, Japan) at 263 nm. Drug release mechanism was determined by Zero order and First order plots [8].
The accelerated stability studies were performed as per the ICH guidelines. Selected formulations of Dicloxacillin sodium were packed in aluminum pouch and subjected to short term stability at 25 °C / 60% RH and accelerated stability at 40°C / 75% RH for a period of 3 months. Samples from each formulation which are kept for examination were withdrawn at definite time intervals. The withdrawn samples were tested for hardness, in vitro buoyancy and assayed for drug content and in vitro drug release [9].
In the present study, a total of 15 formulations of gastro retentive floating tablets of Dicloxacillin sodium were prepared by wet granulation technique using different polymers like HPMC K100M, Xanthan gum, Na CMC and MCC as semi synthetic and natural polymers, using sodium bicarbonate and citric acid as gas generating agents, lactose anhydrous is used as diluent, starch is used as a binding agent, and magnesium sterate and talc as lubricants. Formulations were optimized by different ratios of polymers.
Precompression parameters of Dicloxacillin sodium are shown in Table 2. The bulk density of the formulation ranged between 0.408 ± 0.004 g/mL and 0.578 ± 0.010 g/mL. Tapped density varied between 0.456 ± 0.001 g/mL and 0.675 ± 0.026 g/mL. Carr’s index value ranged between 9.23 ± 0.122% to 16.98 ± 0.274%. Hausner ratio was found between 1.10 ± 0.001 and 1.20 ± 0.003 and Angle of repose has been used as indirect method of quantifying power flow ability, and fallen between 25.67 ± 1.111 to 28.85 ± 0.439. Pre-compression parameters play an important role in improving the flow properties of pharmaceuticals especially in tablet formulation. These include bulk density, tapped density, Carr’s index, Hausner ratio and Angle of repose. Before formulation of floating tablets, the drug and ingredients were evaluated for all the above said parameters and it was found that all the observations were within the prescribed limits of IP. All the formulations were fallen in good flow character based on angle of repose, compressibility index and Hausner ratio reports.
Post-compression parameters of Dicloxacillin floating tablets are showed in Table 3. Weight variation of floating tablets ranged from 649.6 ± 1.349 to 651.7 ± 1.567. Thickness ranged between 5.605 ± 0.036 mm and 6.127 ± 0.044 mm. The diameter varied between 12.004 ± 0.059 mm and 12.133 ± 0.032 mm. The hardness lies between 5.24 ± 0.164 and 5.91 ± 0.109. The friability of all gastro retentive floating tablets of Dicloxacillin sodium was found between 0.263 ± 0.002 and 508 ± 0.002. Drug content ranged between 96.92 ± 0.627 and 98.79 ± 0.242.
The average weights were found to be within (± 7.5) the prescribed official limits. The thickness of the floating tablet indicated that die fill was uniform. The thickness depends upon the size of the punch (12 mm) and the weight of the tablet (650 mg). Friability is needed for tablets to withstand force of compression applied during the manufacture of tablets and all the formulated floating tablets of Dicloxacillin sodium were shown the percentage friability within the official limits (i.e. not more than 1 %). Formulations showed favourable drug content which were within the limits of specifications.
The in vitro buoyancy properties (floating lag time and total floating time) of prepared gastro retentive floating tablets of Dicloxacillin sodium were showed in Table 4. All formulations showed floating lag time between 32.27 ± 0.510 to 96.32 ± 1.618 sec. Formulation F14 showed floating lag time of 34.09 ± 1.154. Formulations F1 – F3 were prepared using different drug to polymer ratios (Drug: HPMC K100M; 280:200, 280:240 and 280:290 mg). Formulations F4 – F6 were prepared using different drug to polymer ratios (Drug: Xanthan gum; 280:200, 280:240 and 280:290 mg). Formulations F7-F9 was prepared using different drug to polymer ratios (Drug: Na CMC; 280:200, 280:240 and 280:290 mg). Formulations F10 – F12 were prepared using different drug to polymer ratios (Drug: MCC; 280:200, 280:240 and 280:290 mg). Formulations F13 – F15 were prepared using different drug to polymer combination ratios (Drug : HPMC K100M : Xanthan gum; 280: 150 : 100, Drug : HPMC K100M: Na CMC; 280 : 150 : 100mg and Drug : HPMC K100M : MCC; 280 : 150 : 100 mg) all formulations were containing gas generating agent (combination of sodium bicarbonate and citric acid). Floating lag time varied by different polymers and polymer ratios. This showed that as the polymer concentration increased floating lag time decreased and total floating time increased.
The percentage water uptake of prepared gastro retentive floating tablets of Dicloxacillin sodium were shown in Table 5. The swelling indices were increased with increase in polymer concentration. Formulations containing HPMC K100M and Na CMC showed higher swelling indices as compared with other formulations containing the same amount of Xanthan gum and MCC. This may be due to the formulations containing variable concentrations of HPMC K100M and Na CMC formed a viscous gel layer during the dissolution. It was observed that combination of HPMC K100M and Na CMC showed maximum swelling. Swelling index values starts decreased when polymer erosion starts in medium.
The cumulative drug release of the different formulations F1 – F15 were carried out by the procedure mentioned earlier. The formulations are carried out for the release studies for about 12 h. The release rates obtained for the formulations mentioned above are 96.86 ± 0.751, 95.72 ± 0.712, 97.18 ± 0.572, 96.39 ± 0.067, 92.71 ± 0.125, 96.89 ± 0.051, 91.96 ± 0.093, 95.96 ± 0.093, 95.07 ± 1.019, 96.78 ± 0.010, 92.31 ± 0.575, 95.06 ± 0.472, 94.98 ± 0.101, 96.46 ± 0.552 and 94.81 ± 0.102 respectively. The results obtained proved that the in vitro release is influenced by the polymer ratios. Because as the mentioned in previously increasing polymers concentration the more gel layer will form around the tablet and sustains the release of the drug from the tablet. The release rates obtained are shown in Fig. 1 and Fig. 2. It has been concluded that formulation proposed with high polymer concentration showed sustain release of the drug up to 12 h.
From the release profile formulation F4 is selected as the best formulation. The r2 and k and n values are given in Table 6. Based on the r2 values obtained the mechanism of drug release was determined. From the r2 values it was fitted to zero order kinetics.
During and at the end of the accelerated stability, the tested tablets showed non-significantly different drug content from that observed at the beginning of the study. They also showed satisfactory hardness and buoyancy properties during and at the end of the accelerated study period. The selected formulation of Dicloxacillin sodium floating tablets were carried out for stability studies for 3 months in different temperatures such as short term stability at 25 ± 2 °C / 60 ± 5% R.H and accelerated stability at 40 ± 2 °C / 75 ± 5% R.H for a period of 3 months and the samples were tested for hardness, in vitro buoyancy, drug content and in vitro drug release for every month and results were shown in Table 7. There was no significant change in the hardness, in vitro buoyancy, drug content and in vitro drug release of the Dicloxacillin sodium floating tablets.
Gastroretentive floating tablets of Dicloxacillin sodium were developed to overcome the less half life and subsequent frequent dosing. In vitro studies and water uptake studies have shown that this is a potential drug delivery system for Dicloxacillin sodium with a good stability and sustain release profile. From in vitro release studies and water uptake study it was a good stability and sustains the release of drug.