e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
Department of Pharmaceutics, TVES’s Honorable Loksevak Madhukarrao Chaudhari College of Pharmacy, Faizpur, India
Received: 03/12/2013 Accepted: 28/12/2013
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
The aim of the present work is to develop the Metoclopramide hydrochloride microsphere using Eudragit RL 100 and hydroxyl propyl methyl cellulose (HPMC K100) as a polymer by solvent evaporation method for Sustained effect. For the preparation of Metoclopramide Hydrochloride Microsphere the solvent system i.e., (Dichloromethane and ethanol) and the drug–polymer ratio are use in various concentrations, to obtain the desire sustained formulation. Various formulation of metoclopramide hydrochloride microsphere was formulated by using Eudragit RL 100 and HPMC K100 polymers. The microsphere was evaluated for physical characterizations angle of repose, particle size, drug entrapment efficiency, in-vitro dissolution. Results of all the physical and in-vitro dissolution data concluded in that formulation E-6 was the most promising formulation. The E-6 batch microsphere prepared from the Eudragit RL100 polymer in that the drug-polymer ratio is 01:1.5, and 01:01 Solvent system (DCM: Ethanol), using 2% span 80 as dispersing agent. Metoclopramide hydrochloride microsphere E-6 formulation releases the maximum drug i.e., 95.87 ± 0.70 for 12 hrs. The kinetic study was carried out and the best fitted kinetic model for E6 optimised batch was Korsmeyer peppas have R value 0.998 and k value was 13.62.
Microspheres, solvent evaporation method, Eudragit RL 100,HPMCK 100M.
Drugs that are easily absorbed from the gastrointestinal tract (GIT) and have a short half life are eliminated quickly from the blood circulation; require frequent dosing [1,2]. To avoid this problem, the oral sustained release (SR) formulations have been developed in an attempt to release the drug slowly into the GIT and maintain a constant drug concentration in the serum for longer period of time [3]. Metoclopramide hydrochloride is potent Anti-emetic and prokinetic, effective even for preventing emesis induced by cancer chemotherapy. It is also used in certain disorders of digestive tract, including gastriostasis and gastroesophagal reflux. Metoclopramide hydrochloride have oral bioavailability of around 75% so oral administration is suitable route, but its plasma half life is short that is around 3-5 hours, so repeated administration is required hence the development of sustained release dosage form would clearly be advantages to reduce dosing frequency [4,5]. Microspheres have been widely accepted as a means to achieve oral sustained release. The microsphere requires a polymeric substance as a coat material or carrier. A number of different substances both biodegradable as well as non-biodegradable have been investigated for the preparation of microsphere. It not only reduces the dose of the drug, reaching to the effective biological sites rapidly but also results in reduced toxicity [6]. From the literature survey it revealed that for Metoclopramide hydrochloride the Eudragit RL100 and HPMC K100 was not studied for microsphere in different ratio using the dichloromethane and ethanol as Solvent system in different ratios. Hence, the aim of the present work was to develop the Metoclopramide hydrochloride microsphere by using polymer such as Eudragit RL 100 and hydroxyl propyl methyl cellulose (HPMC K100) for Sustained effect of Metoclopramide hydrochloride. For the preparation of Metoclopramide Hydrochloride microsphere the solvent Dichloromethane and ethanol is use in various ratio to obtain the desire sustained formulation.
Metoclopramide Hydrochloride was obtained as gift sample by Haffkin Pharma. Jalgaon. India. HPMC-K100M was obtained as a gift sample from Wockhardt Pvt. Ltd, Aurangabad.India. Eudragit RL 100 was obtained as a gift sample from Evonik Pvt Ltd, mumbai. All other materials and solvents used were of analytical grade.
The Eudragit RL 100 microspheres and Hydroxy Propyl Methyl Cellulose (HPMC) K 100 microsphere were prepared by solvent evaporation method using dichloromethane (DCM) and ethanol as the solvent in appropriate ratio. Weighed quantity of Polymer and Metoclopramide Hydrochloride was added into the solvent system. The resulting solution was added drop wise into continuously stirring 100 ml light liquid paraffin containing 2.0 %v/v of Span 80. The resulting emulsion was stirred using Remi lab stirred at 1000 RPM for 4 hours to facilitate solvent evaporation. The microspheres were finally washed with n-Hexane and dried at room temperature. When the speed of stirrer was less than 1000 or more than 1000 the desire size and shape of microsphere was not obtained.
The microsphere were prepared by using metoclopramide hydrochloride as a drug and Eudragit RL100 and HPMC K100 as polymers, with in the ratio 1:1, 1:1.5 and 1:2 by using dichloromethane and ethanol as solvent system in various concentration, that are mention in Table No.1 and 2. Microsphere was not obtained when the HPMC K100 polymer used in the ratio of 01:01(Drug: Polymer) taken.
Angle of repose is defined as the maximum angle possible between the surface of pile of powder and horizontal plane. The angle of repose for the microsphere of each formulation was determined by the funnel method. The granules mass was allowed to flow out of the funnel orifice on a plane paper kept on the horizontal surface, this forms a pile of granules on the paper. The relationship between angle of repose and flowability were shown in Table No.6. The angle of repose was calculated by substituting the values of the base radius ‘R’ and pile height ‘H’ in the following equation [7].
Tanθ = H /R
Where, H = Pile Height.
R = Radius of Pile
Therefore; 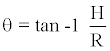
Both loose bulk density (LBD) and tapped bulk density (TBD) were determined. A quantity of 2g of granules from each formula was lightly shaken to break agglomerates if any and then was introduced into a 10ml-measuring cylinder. It was allowed to fall under its own weight onto a hard surface from the height of 2.5cm at 2- second intervals. The tapping was continued until no further change in volume was noted. Loose bulk density (LBD) and Tapped bulk density (TBD) were calculated using the following formulae [8,9].
LBD = Weight of the granules/Volume of the packing
TBD = Weight of the granules/Tapped volume of the packing
The compressibility indices of the formulation blends were determined using Carr’s compressibility index formula.
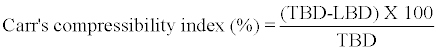
Hausner’s ratio of microparticles was determined by comparing the tapped density to the bulk density using the equation.
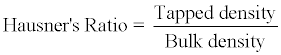
The particle size was measured using a Stage micrometer, and the mean particle size was calculated by measuring 200 particles with the help of a calibrated stage micrometer. A small amount of dry microspheres was suspended in liquid paraffin (10 ml). A small drop of suspension thus obtained was placed on a clean glass slide. The slide containing microspheres was mounted on the stage of the microscope and diameter of at least 100 particles was measured using a calibrated optical micrometer [10].
The percentage yield of different formulations was determined by weighing the microspheres after drying. percentage yield was calculated as follows [11].
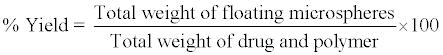
The various batches of the microspheres were subjected to estimation of drug content. The microspheres equivalent to 20 mg of Metoclopramide Hydrochloride, were accurately weighed and crushed. The powdered of microspheres were dissolved in distilled water in volumetric flask and the volume is adjusted with distilled water upto 100 ml (200 μg/ml). This solution is then filtered through Whatmann filter paper. After filtration, from this solution accurate quantity 0.5 ml was pipette out and diluted up to 10 ml (10 μg/ml) with distilled water and the absorbance was measured at 272 nm against Distilled water as a blank [12].
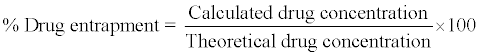
From the formulated batches of microspheres, formulation E6 was examined for surface morphology and shape using scanning electron microscope. Fig. No. 19-21. Morphology details of the specimens were determined by using a scanning electron microscope (SEM), Model JEOL 5400, Japan. The samples were dried thoroughly in vacuum desicator before mounting on brass specimen studies. Sample was fixed on carbon tape and fine gold sputtering was applied in a high vacuum evaporator. The acceleration voltage was set at 20KV during scanning. Microphotographs were taken on different magnification at 50X, 100X, and 500X was used for surface morphology [13].
The Fourier Transform Infra-Red analysis was conducted for the analysis of drug polymer interaction and stability of drug during microencapsulation process. The FT-IR spectra were obtained using FT-IR spectrometer (Shimadzu). Spectrum of pure Metoclopramide hydrochloride, Eudragit RL 100, and HPMC K100 and mixture of these compound were previously ground and mixed thoroughly with potassium bromide, an infrared transparent matrix in 1:5 (sample : KBr) ratio, respectively. The KBr discs were prepared by compressing the powders by applying a pressure [14].
The DSC measurements were performed on a Extar DSC 6220, Japan differential scanning calorimeter with thermal analyzer. All accurately weighed samples (about 5 mg of MCP, Eudragit RL 100, and HPMC K100) were placed in a sealed aluminium pans, before heating under nitrogen flow (20 ml/min) at a scanning rate of 10°C per min from 25 to 200°C. The temperature range used was 0 –600°C. An empty aluminium pan was used as reference [15,16].
The In-Vitro dissolution studies of the sustained release Microsphere formulation of Metoclopramide Hydrochloride were carried out using dissolution test apparatus USP-I Basket type. Weighed amount of microspheres equivalent to 10 mg to the total weight of drug-polymer used in microsphere formulation, they were packed in musclin cloth and placed in the basket. The dissolution medium consisted of 900 ml of standard buffer of pH 1.2 for the first 2 hours, followed by pH 6.8 for the remaining time period up to 8 to 12 hours. The temperature of the medium was maintained at 37±0.50C. The speed of rotation of the basket was kept at 100 rpm. Aliquots of 10 ml were withdrawn after every half an hour for the first two hours and thereby every hour for a total of 12 hours. The samples so withdrawn were replaced with the fresh dissolution medium equilibrated at the same temperature. The drug released at the different time intervals from the dosage form is measured by U.V. visible spectrophotometer, by measuring the absorbance for the samples solutions at 272 nm for Metoclopramide Hydrochloride. The dissolution characteristics of each samples was studied, after accounting for loss in the initial concentration of the drug – Metoclopramide hydrochloride while changing the buffer. The release studies for each formulation were conducted in triplicate, indicating the reproducibility of the results. Table No. 8 shows operational parameter constants, and Table No.17-21 shows the % cumulative release from the microsphere [17].
To analyze the mechanism of release and release rate kinetics of the dosage form, the data obtained were fitted into Zero-order, First-order, Higuchi matrix, Peppas and Hixson Crowell model using PCP-DISSO -v3 software. Based on the r-value, the best-fit model was selected [18-20].
The stability study of optimized formulation E-6 was carried out at for 100 days. The microspheres were individually wrapped using aluminium foil and packed in ambered colored screw capped bottle and kept at above specified condition in incubator for a period, and analyzed at 0, 25, 50, 75, and 100 days for their changes in various physical properties [21].
Angle repose of Eudragit RL100 microspheres was observed in range of 23°.61’- 29°.62’ i.e. less than 30. While the microsphere prepared by the polymer HPMC K100, there Angle of repose was determined and was observed in the range of 31°.10’-34°.13’. The bulk density value of different batches of Eudragit RL100 microspheres was determined, the Bulk density was ranged from 0.437 ± 0.03 - 0.476± 0.01 gm/cm3 and Tapped density was ranged from 0.528 ± 0.03 – 0.597 ± 0.03 g/cm2. The Bulk density was ranged from 0.508 ± 0.03 - 0.623± 0.01 g/cm2 and Tapped density was ranged from 0.539±0.02 – 0.683±0.01 g/cm2 of different batches of HPMC K100 microsphere respectively. The percentage compressibility index values for Eudragit RL100 was ranged between 13.60 % - 19.96 %. The percentage compressibility index values for HPMCK 100 was ranged between 20.01% - 26.64%.
The average particle size of the microspheres was calculated and lies between 135.33-389.66 respectively. The percentage yield of different batches was determined by weighing the microspheres after drying. The percentage yields of different formulation of Eudragit RL100 microsphere were in range of 67.92 - 78.30% and the percentage yield of HPMC K100 microsphere was in range of 56.78% - 66.20% as shown in table No.4. The percentage yield of microspheres appeared unchanged by changing polymer ratio. The drug entrapment efficiency of different batches of microspheres was determined, for Eudragit RL100 the entrapment efficiency was in the range of 49.37% - 83.12%. For HPMC K100 was in the range of 41.56% - 68.12% as shown in Table No.16. Drug entrapment efficiency was decreased when the solvent ratio of dichloromethane and ethanol changes, 1:1 ratio of solvent shows the maximum drug entrapment as compared to (1:2, 1:3, 2:1, 3:1) ratios.
The size and surface morphology of microspheres were examined by scanning electron microscopy as shown in figures (Fig No.1) illustrating the microphotographs of formulation E6 at lower and higher magnification. The microspheres were spherical with no visible major surface irregularity. Few wrinkles and inward dents were appeared at the surface of microsphere. It may due to collapse of microspheres during the drying process.
The surface morphology of both formulations was examined at higher magnification (500X) which illustrates the smooth surface of microspheres. Some small pores and cavities were present on the surface of microspheres, probably arising as a trace of solvent evaporation during the process.
The FT-IR spectra of Metoclopramide hydrochloride, Eudragit RL100, HPMC K100 and physical mixture of drug-polymer and microspheres of E6 batch were recorded shown in Fig. No.2-4. the drug, Metoclopramide hydrochloride present in the formulation E6 was confirmed by FT-IR spectra. The characteristics peaks due to C=O stretching of amide, C=C stretching aromatic, C=H stretching of alkenes, N-H stretching of amine, C-N stretching groups present metoclopramide hydrochloride appeared in microspheres spectra of E6 Formulation, without any remarkable change in their position after successful encapsulation, indicating no chemical interaction between drug and polymers.
The differential scanning Calorimetry of the metoclopramide hydrochloride, Eudragit RL100, and the E6 formulation batch was recorded as shown in Fig No. 5. The DSC thermograms of E6 formulation confirmed that there is no interaction between drug and polymers as shown in Fig.no 5. It also showed a reduction in intensity of the peak and there is no new peaks found and endothermic to exothermic change not occure. Hence, it was confirmed that there was no interaction between drug and excipients.
The microsphere formulation of metoclopramide hydrochloride containing Eudragit RL100 and HPMC K100 polymers, in-vitro drug release study carried out. Drug release from the metoclopramide hydrochloride containing Eudragit RL100 microsphere, is between 9–12 hrs gives sustained release effect. While the drug release from the HPMC K100 is between 5-7 hrs. From the in-vitro drug release study it is clear that the drug release from polymer containing Eudragit RL100 gives good release as compared to polymer containing HPMC K100.
Drug release rate is affected by changing the solvent ratio of DCM and ethanol, and also affected by the changing the drug polymer concentration. The solvent use in equal proportion i.e., 01:01 shows good drug release as compare to (01:2, 01:3, 02:1, 03:01) ratios. Increase in DCM concentration in microspheres formulation decrease in release rate, while increase in concentration ethanol slightly affected to the release rate of microsphere formulation. The formulations containing drug-polymer concentration 01:1.5 shows good release rate as compared to 01:01 and 01:02. As the concentration is increase i.e. 01:2.5 and 01:03, the microsphere was not obtained. HPMC K100 microsphere prepared from 01:01 Drug-polymer concentration was not obtained.
The in-vitro release data was applied to various kinetic models to predict the drug release kinetic mechanism and are shown in Table No.5. The best fit model for the optimised batch (E6) formulation is the Korsmeyer Peppas, having R value 0.998 and K value is 13.62
Accelerated stability studies (AST) was carried for optimized formulation E6 by exposing it to 40°C/75% RH for one month and analyzed the sample at the interval of 25, 50, 75, and 100 days shown in Table No.24. The Colour, % Drug content efficiency and % cumulative release was calculated. And finally the SEM was studied after 100 days; the microspheres were spherical with no visible major surface irregularity. Few wrinkles and inward dents were appeared at the surface of microsphere. It may due to collapse of microspheres during the drying process. There was no change in the Surface morphology of microspheres.
The aim of the present work was to develop the Metoclopramide hydrochloride microsphere by using polymer such as Eudragit RL 100 and hydroxyl propyl methyl cellulose (HPMC K100) for Sustained effect of Metoclopramide hydrochloride. For the preparation of Metoclopramide Hydrochloride microsphere the solvent Dichloromethane and ethanol is use in various ratio to obtain the desire sustained formulation. Metoclopramide hydrochloride microsphere E-6 formulation releases the maximum drug i.e., 95.87 ± 0.70 for 12 hrs. While other formulation batches does not shows the Proper release pattern as compare to E6 formulation. Hence the E6 formulation is best formulation on the basis of release pattern. The kinetic study was carried out and the best fitted kinetic model for E6 optimised batch was Korsmeyer peppas have R value 0.998 and k value was 13.62.