e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
Ascent Pharmaceuticals Inc. Research & Development Department, 550 S. Research Place, Central Islip, NY, USA.
Received: 10/09/2014; Revised: 18/09/2014; Accepted: 22/09/2014
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
The objective of fast disintegrating drug delivery systems is to enhance absorption and bioavailability while improving patient compliance. Somehow, most of the existing fast disintegrating drug delivery systems are compromised with one or more quality attributes including patient compliance, therapeutic effect and manufacturing method. This research work attempts to develop a fast disintegrating drug delivery system with multidimensional attributes to improve patient compliance and manufacturing process while enhancing absorption and bioavailability. A fast disintegrating Melt in Mouth Disc (MMD) was developed to enhance absorption via GI tract and oral mucosa. Loratadine was selected as a model drug for the study. MMD was developed by a direct compression method using a rotary compressed tablet machine using water soluble and insoluble diluents, and super disintegrants. The relationship between input materials, critical processing parameters and critical quality attributes was established through Quality by Design using a 23 factorial design. Results demonstrated that ratio between water soluble/insoluble diluents, and type of super disintegrants, significantly affect the response variables. The compressed thin tablets were characterized for uniformity of weight, hardness, disintegration time, dissolution, and wetting time. Optimized formulation exhibited a hardness of 5.0 N, disintegration time <20 seconds, wetting time < 25 seconds, and drug release 99% within 5 minutes.
Loratadine, Mannitol, Silicified Microcrystalline cellulose, Rapid disintegrating compressed Discs
Despite a rapid growth in the development of new drug delivery systems, oral delivery is still the most preferred route because it is considered the safest, convenient and most economical route[1-2]. Among all novel drug delivery systems orally disintegrating dosage forms such as tablets, films, and soft chews are one of the most commercially successful oral solid dosage forms [3]. Fast disintegrating or fast dissolving tablets are the line extension and newer generation of traditional solid compressed tablets. According the US Food and Drug Administration's Center for Drug Evaluation and Research, Orally Disintegrating Tablets (ODT) are “A solid dosage form containing medicinal substances which disintegrates rapidly, usually within a matter of seconds, when placed upon the tongue.” It also lists the recommended attributes of ODT dosage forms: low tablet weight, small tablet size, highly soluble components, and rapid disintegration [4]. These attributes, which help to address draw backs and improve patient compliance like difficulty in swallowing (dysphasia) and chewing in some patients particularly in geriatric and pediatric patients [5-6]. The general methodology for manufacturing ODT is compression and lyophilization. Most of the ODT made with a compression method may not be within purview of FDA guidelines, and degree of disintegration and/or dispersion rate depends on hardness and amount and type of water soluble and super disintegrates employed in the formulation whereas, lyophilization technology is more complicated and needs special equipment [7-9]. In addition to these two technologies, thin-film technology is also popularized mainly in OTC products and industry considering it is an alternative route for ODT [10]. The main setback for this thin strip technology is special packaging and high dose drugs can’t be accommodated [11]. The patient compliance, ease of manufacturing, and extended shelf life of the molecule and FDA guidelines warranted the development of new methods for ODT. These make inroads to search for an alternative technology for ODT that are Melt in Mouth Discs. By considering the new FDA regulatory implications, FDA guidelines and patient compliance, the author is proposing ODT with low tablet weight (< 60 mg), fast disintegration time (<20 seconds), soluble ingredients (Mannitol, lactose) and size (< 10 mm) in thin disc dosage form by a simple rotary tablet compression technique.
Rationale for proposing MMD as an alternative to existing ODTs is to develop a manufacturing process for low dose and high potent drugs using standard rotary tablet compression equipment. Furthermore, it reduces excessive usage of excipients in the formulation and subsequently reduces unpleasant taste and grittiness in the mouth. Using MMD technology tablets can be produced as thin as oral films with similar and better physical attributes and stability.
The model drug was Loratadine. Loratadine is an antihistamine, it is chemically ethyl 4-(8-chloro-5,6-dihydro-11H-benzo [5,6] cyclohepta[1,2-b]pyridine-11-ylidene)-1-piperidinecarboxylate, molecular weight 382.88, molecular formula C22H23ClN2O2 (Figure 1). It is an odorless white crystalline solid or white powder with a bitter taste. Lipophilic and non-ionizable forms of Loratadine are freely soluble in oils like mono- and di-glycerides of capric and caprylic acids (Capmul® MCM). Loratadine is insoluble in water (1.1 x 10-5 mg/mL), but very soluble in acetone, methanol, toluene and chloroform. The solubility of Loratadine in different pH media varied significantly with gastrointestinal tract pH range from 1.2 to 7.5 [12].
The Melt in Mouth Discs (MMD) contains Loratadine, super disintegrant like, crosspovidone, crosscaramellose, silicified microcrystalline cellulose powder (carrier), mannitol powder, lactose, (water soluble diluent), magnesium stearate, (lubricant), sucralose, (sweetener), and orange flavor.
Initial screening formulation trials were performed using experience gained on similar type of formulations and information obtained from public domain. One prototype formulation was selected from eight prototype formulations for further optimization. A 32 full factorial design was employed, containing two independent variables for the optimization of Loratadine Melt in Mouth Discs. The concentration of superdisintegrant (X1), and ratio between water soluble and insoluble diluent (X2) were selected as independent variables at two levels. The disintegration time, wetting time, hardness and friability were selected as dependent variables [13]. The experimental details are given in Table 1, 2 and 3.
Melt in Mouth compressed Loratadine discs, 10 mg were prepared by using a direct compression method. Direct compression was carried out by prescreening all inactive ingredients through # 30 screen followed by geometric mixing in double cone blender for 10 minutes at 23 ± 2 rpm. Finally, the blend was lubricated with Magnesium stearate for 5 minutes in double cone blender at 23 ± 2 rpm. The resultant flavored Loratadine MMD blend was compressed into thin discs using DB16 rotarty station compression machine using 6 mm flat round punches with tablet weight of 55 mg.
Loratadine MIMD final blend was characterized for density (true, bulk and tapped density), carr’s index, Hausner ratio, angle of ratio, moisture content and sieve analysis. The compressed Loratadine discs were evaluated for appearance, thickness, hardness (Dr.Schleuniger Pharmatron – Tabstat 2000), disintegration time (texture analyzer (TA), model CS225, Chatilon force measurement (Manufacturer: Ametek). Dissolution testing was performed using an USP apparatus II at 50 rpm in 900 mL 0.1 N HCl. and the results are reported as percent weight/weight compared to the USP Reference Standard <711>, with the amount equivalent to the 10 mg label claim.
Bulk density of Loratadine MMD blend was determined by placing known amount of mass in graduated measuring cylinder. The bulk density of the sample is expressed in g/mL and is expressed as
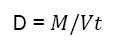
Where, M is the mass of the sample and Vt is the volume of the sample
Tapped density of the blend is the average mass per unit volume and is determined by mechanically tapping a measuring cylinder containing known amount of sample on mechanical tapping apparatus. The tapped volume was measured by tapping the powder to constant volume. The tapped density of the sample is expressed in g/mL and is expressed as
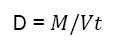
Where, M is the mass of the sample and Vt is the final volume of the sample after tapping
Tapped density was determined by using Stampf volumeter STAV2003 (manufacturer: J. Englesmann A.-G). Samples were weighed in a tared 25 mL graduated cylinder and mounted on the Stampf volumeter. The samples were subjected to 25 taps and the powder volume was visually determined. Tapping and volume determination was repeated until a constant reading was observed for three to four measurements.
Carr’s index and Hausner ratio were used to describe the compressability and flow properties of the powder. The bulk and tapped density values were used to calculate the Carr’s index and Hausner ratio to evaluate the flow and compression characteristics of granules. The Carr’s index calculated by the following formula:
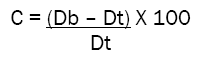
Th Hausner’s ratio calculated by the following formula:
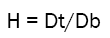
Where C = Carr’s index, Db =Bulk density, and Dt = Tapped density
Lower Hausner’s ratio (< 1.25) indicates better flow properties and high Hausner’s ratio (> 1.25) indicates poor flow.
The flow property of the blend was measured by according to USP method. According to USP method, the angle of repose is the angle formed by the horizontal base of the bench surface and the edge of a cone-like pile of granules. Funnel used was a stainless steel funnel and the size of the orifice was 10 mm and the height from the beginning of funnel to end of orifice was 111 mm. The funnel was fixed in place, 4 cm above the bench surface. After the cone from 5 g of sample was built, height of the granules forming the cone (h) and the radius (r) of the base were measured. The angle of repose (θ) was calculated as follows:
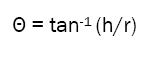
Particle size distribution was performed for the blend using an ATM Sonic Sifter and stacked US Standard Screens. Sifting was for 10 minutes, at amplitude six (6), using the pulse function.
Post compression characterization of tablets: appearance, disintegration time, hardness, friability, thickness, and weight variation were evaluated using following test methods.
Disintegration time of compressed discs was determined using texture analyzer (TA), model CS225, Chatilon force measurement (Manufacturer: Ametek). The instrument is calibrated with 5 kg load cell and fitted with 1 cm2 S.S. flat faced probe. Proper disintegration test entails that the tablet be attached to the flat-bottomed probe with a double-sided adhesive tape. Subsequently, the probe will move until a trigger force is encountered at which point, the TA is configured to maintain a pre-determined nominal force for a set period of time (120 seconds). As the tablet begins to disintegrate the TA will appropriately measure the distance of penetration as the compressed tablet is submerged in the medium (Figure 2).
Hardness of compressed discs was determined by using tablet hardness tester Dr.Schleuniger Pharmatron – Tabstat 2000. The results are expressed as mean value ±SD.
Friability test for discs were determined using according to method described in USP. Randomly selected 10 discs were dedusted and weighed and determined total mass lost by approximately 10 discs after 100 rotations at 25 rpm for four minutes. The amount of mass loss was calculated using below formula and friability value presented as percentage of weight by weight.
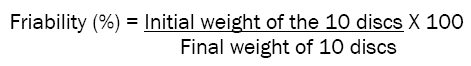
Weight variation of compressed discs was determined quantitatively by weighing 20 discs individually and together. In-process limit for weight variation is ± 5% of theoretical wafer weight.
Weight Variation is calculated using below formula
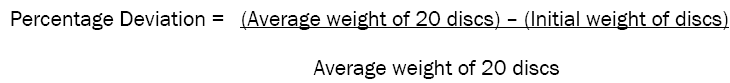
Wetting time of the fast dissolving disc was determined by placing sample with FD&C Blue # 1 in petri plate containing 15 mL water with filter paper. The wetting time for the disc was calculated as the time required becoming upper surface of the tablet completely blue.
The dissolution of Loratadine Discs was carried out in Apparatus II, in the dissolution medium, 500 mL 0.1 N Hydrochloric acid at 37 ± 1C at 50 rpm. Test was run for 30 minutes with sampling point 5, 10, 15 and 30 minutes and its absorbance was measured at 265 nm.
Loratadine fast disintegrating Melt in Mouth Discs were successfully developed and manufactured within FDA purview employing direct compression technology. A partial Quality by Design (QbD) approach applied to optimize the formulation using crosspovidone as superdisintegrant, water soluble and insoluble diluents on equal ratio are used as variables. These variables were identified from prototype formulations. A total of eight formulations were designed to identify the influence of variables on attributes of the finished product. Results are given in Table 4 and 5. Preliminary screening experiments (LD-01 to 08) were formulated to identify the significance of each ingredient on physical and chemical attributes of the finished drug product. For each formulation, pre and post compression characterization was performed as described earlier. Pre-compression characterization was performed on blends including flow parameters such as angle of repose, density such as bulk and tapped, and compressibility index using Carr’s index and Hausner’s ratio.
Throughout study, tablet weight (55 mg) and drug concentration (18.18%) were kept constant. Preliminary screening samples were prepared with two different superdisintegrants crosspovidone and crosscaramellose alone and in combination at a concentration of 25.82% w/w. Total concentration of water soluble and insoluble diluents mannitol powder, lactose and silicified microcrystalline cellulose powder in the formulation is around 54.85% w/w. Other excipients sucralose, orange flavor and magnesium stearate were kept constant.
All formulations showed acceptable bulk and tapped density in the range of 0.4054 g/mL to 0.5405 g/mL and 0.5113 g/mL to 06146 g/mL respectively. The angle of repose observed in the range of 28.05° to 32.05 indicating all formulations have excellent flow properties except LD-2 and LD-5 have good flow properties. Carr’s index and Hausner’s ratio were in the range of 14.20 to 18.12 and 1.17 to 1.20 indicating all formulations have good compressibility index. High amount of lactose containing formulations showed high bulk and tapped densities compared to other formulations. Formulation LD-4 showed good compressibility index and flow index compared to other formulations.
The powder blends were compressed into tablets using 16 station compression machine with 6 mm round flat faced punches. The compressed tablets were evaluated for physical properties as described earlier. All blends were compressed with constant compressional forces to evaluate the critical attributes thickness and disintegration time. The target compressional force was between 5.0 N and 6.0 N. Formulations LD-2, -3, -4, -7, & 8 showed poor wettability and higher disintegration time in the range of 71 seconds to 92 seconds, it could be due to formation of high compacted mass during tableting. Formulations containing equal ratio of water soluble and insoluble diluents showed good wettability and faster disintegration time. Formulation LD-4 disintegrated in less than 30 seconds with less friability (<0.01%). Based on preliminary screening experiments, formulation LD-4 was selected for further optimization.
Formulations OP-7 and OP-8 exhibited good flow index, bulk and tapped density 26.35° and 28.05°, 0.4994 g/mL and 05204 g/mL and 05096 g/ml and 0.5398 g/mL respectively. Carr’s index found to be 14.12 and 15.00 for OP-7 and OP-8 respectively. All formulation pre-compression attributes are given in Table 6.
The compressed discs were evaluated for appearance, weight variation, hardness, wetting time, and dissolution (Table 7). Formulations OP-7 and OP-8 showed all acceptable quality attributes, weight variation found to be within 0.2 SD, hardness found to be in the range of 5.1N and 5.2 N respectively. Formulations OP-7 and OP-8 exhibited wetting time disintegration time less than 30 seconds compared to other optimization batches. Irrespective of concentration of superdisintegrants and ratio between water soluble and insoluble diluents quantity per disc showed above 99% drug release in five minutes.
From this study, it can be concluded that fast disintegrating Melt in Mouth Discs can be considered as an alternative dosage form and can be manufactured using a standard rotary compression machine with excellent physical attributes. Fast disintegrating Melt in Mouth Discs were successfully developed and manufactured within the FDA guidelines. Furthermore, fast disintegrating Melt in Mouth Discs can be used as sublingual tablets to enhance the oromucosal absorption where the drugs have first pass metabolism. It was also observed that Melt in Mouth Discs dosage forms reduce excipient cost, manufacturing cost, grittiness in the mouth and moreover, good blend uniformity obtained with low dose and highly potent drugs.